Mettl3‑mediated m6A RNA methylation regulates osteolysis induced by titanium particles
- PMID: 38214327
- PMCID: PMC10823336
- DOI: 10.3892/mmr.2024.13160
Mettl3‑mediated m6A RNA methylation regulates osteolysis induced by titanium particles
Abstract
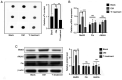
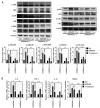
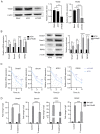
Peri‑prosthetic osteolysis (PPO) induced by wear particles is considered the primary cause of titanium prosthesis failure and revision surgery. The specific molecular mechanisms involve titanium particles inducing multiple intracellular pathways, which impact disease prevention and the targeted therapy of PPO. Notably, N6‑methyladenosine (m6A) serves critical roles in epigenetic regulation, particularly in bone metabolism and inflammatory responses. Thus, the present study aimed to determine the role of RNA methylation in titanium particle‑induced osteolysis. Results of reverse transcription‑quantitative PCR (RT‑qPCR), western blotting, ELISA and RNA dot blot assays revealed that titanium particles induced osteogenic inhibition and proinflammatory responses, accompanied by the reduced expression of methyltransferase‑like (Mettl) 3, a key component of m6A methyltransferase. Specific lentiviruses vectors were employed for Mettl3 knockdown and overexpression experiments. RT‑qPCR, western blotting and ELISA revealed that the knockdown of Mettl3 induced osteogenic inhibition and proinflammatory responses comparable with that induced by titanium particle, while Mettl3 overexpression attenuated titanium particle‑induced cellular reactions. Methylated RNA immunoprecipitation‑qPCR results revealed that titanium particles mediated the methylation of two inhibitory molecules, namely Smad7 and SMAD specific E3 ubiquitin protein ligase 1, via Mettl3 in bone morphogenetic protein signaling, leading to osteogenic inhibition. Furthermore, titanium particles induced activation of the nucleotide binding oligomerization domain 1 signaling pathway through methylation regulation, and the subsequent activation of the MAPK and NF‑κB pathways. Collectively, the results of the present study indicated that titanium particles utilized Mettl3 as an upstream regulatory molecule to induce osteogenic inhibition and inflammatory responses. Thus, the present study may provide novel insights into potential therapeutic targets for aseptic loosening in titanium prostheses.
Keywords: N6‑methyladenosine RNA methylation; aseptic loosening; epigenetic regulation; methyltransferase‑like 3; osteolysis; titanium particles.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





Similar articles
-
METTL3‑mediated m6A methylation and its impact on OTUD1 expression in chronic obstructive pulmonary disease.Mol Med Rep. 2025 Aug;32(2):206. doi: 10.3892/mmr.2025.13571. Epub 2025 May 26. Mol Med Rep. 2025. PMID: 40417884 Free PMC article.
-
Genetic and pharmacological activation of Hedgehog signaling inhibits osteoclastogenesis and attenuates titanium particle-induced osteolysis partly through suppressing the JNK/c-Fos-NFATc1 cascade.Theranostics. 2020 May 17;10(15):6638-6660. doi: 10.7150/thno.44793. eCollection 2020. Theranostics. 2020. PMID: 32550895 Free PMC article.
-
METTL3‑mediated m6A modification of Bcl‑2 mRNA promotes non‑small cell lung cancer progression.Oncol Rep. 2021 Aug;46(2):163. doi: 10.3892/or.2021.8114. Epub 2021 Jun 16. Oncol Rep. 2021. PMID: 34132367 Free PMC article.
-
Targeting key RNA methylation enzymes to improve the outcome of colorectal cancer chemotherapy (Review).Int J Oncol. 2024 Feb;64(2):17. doi: 10.3892/ijo.2023.5605. Epub 2023 Dec 22. Int J Oncol. 2024. PMID: 38131226 Free PMC article. Review.
-
METTL Family in Healthy and Disease.Mol Biomed. 2024 Aug 19;5(1):33. doi: 10.1186/s43556-024-00194-y. Mol Biomed. 2024. PMID: 39155349 Free PMC article. Review.
Cited by
-
Regulation of Skeletogenic Pathways by m6A RNA Modification: A Comprehensive Review.Calcif Tissue Int. 2025 Apr 3;116(1):58. doi: 10.1007/s00223-025-01367-9. Calcif Tissue Int. 2025. PMID: 40180675 Free PMC article. Review.
-
Towards Enhanced Osteointegration: A Comparative and In-Depth Study of the Biocompatibility of an Innovative Calcium-Doped Zirconia Coating for Biomedical Implants.J Funct Biomater. 2025 May 22;16(6):191. doi: 10.3390/jfb16060191. J Funct Biomater. 2025. PMID: 40558878 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

