Periodontitis aggravates COPD through the activation of γδ T cell and M2 macrophage
- PMID: 38214520
- PMCID: PMC10878042
- DOI: 10.1128/msystems.00572-23
Periodontitis aggravates COPD through the activation of γδ T cell and M2 macrophage
Abstract
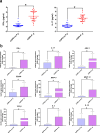
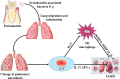
Chronic obstructive pulmonary disease (COPD) is a chronic systemic inflammatory disease with high morbidity and mortality. Periodontitis exacerbates COPD progression; however, the immune mechanisms by which periodontitis affects COPD remain unclear. Here, by constructing periodontitis and COPD mouse models, we demonstrated that periodontitis and COPD could mutually aggravate disease progression. For the first time, we found that the progression was associated with the activation of γδ T cells and M2 macrophages, and M2 polarization of macrophages was affected by γδ T cells activation. In the lung tissues of COPD with periodontitis, the activation of γδ T cells finally led to the increase of IL 17 and IFN γ expression and M2 macrophage polarization. Furthermore, we found that the periodontitis-associated bacteria Porphyromonas gingivalis (P. gingivalis) promoted the activation of γδ T cells and M2 macrophages ex vivo. The data from clinical bronchoalveolar lavage fluid (BALF) samples were consistent with the in vivo and ex vivo experiments. For the first time, our results identified the crucial role of γδ T-M2 immune mechanism in mediating periodontitis-promoted COPD progression. Therefore, targeting at periodontitis treatment and the γδ T-M2 immune mechanism might provide a new practical strategy for COPD prevention or control.IMPORTANCEPeriodontitis exacerbates chronic obstructive pulmonary disease (COPD) progression. For the first time, the current study identified that the impact of periodontitis on COPD progression was associated with the activation of γδ T cells and M2 macrophages and that M2 polarization of macrophages was affected by γδ T cells activation. The results indicated that targeting at periodontitis treatment and the γδ T-M2 immune mechanism might provide a new practical strategy for COPD prevention or control.
Keywords: COPD; IFN γ; IL 17; M2 macrophage; periodontitis; γδ T.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
γδ T Cells Are Required for M2 Macrophage Polarization and Resolution of Ozone-Induced Pulmonary Inflammation in Mice.PLoS One. 2015 Jul 2;10(7):e0131236. doi: 10.1371/journal.pone.0131236. eCollection 2015. PLoS One. 2015. PMID: 26135595 Free PMC article.
-
Hydrogen regulates the M1/M2 polarization of alveolar macrophages in a rat model of chronic obstructive pulmonary disease.Exp Lung Res. 2021 Sep;47(7):301-310. doi: 10.1080/01902148.2021.1919788. Epub 2021 Jul 20. Exp Lung Res. 2021. PMID: 34282696
-
Distribution of γδ and other T-lymphocyte subsets in patients with chronic obstructive pulmonary disease and asthma.Respir Med. 2013 Mar;107(3):413-23. doi: 10.1016/j.rmed.2012.11.012. Epub 2012 Dec 25. Respir Med. 2013. PMID: 23273406
-
Research on the Association Between Periodontitis and COPD.Int J Chron Obstruct Pulmon Dis. 2023 Sep 1;18:1937-1948. doi: 10.2147/COPD.S425172. eCollection 2023. Int J Chron Obstruct Pulmon Dis. 2023. PMID: 37675198 Free PMC article. Review.
-
Macrophages: Their role, activation and polarization in pulmonary diseases.Immunobiology. 2018 Apr-May;223(4-5):383-396. doi: 10.1016/j.imbio.2017.11.001. Epub 2017 Nov 12. Immunobiology. 2018. PMID: 29146235 Free PMC article. Review.
Cited by
-
Environmental exposures, the oral-lung axis and respiratory health: The airway microbiome goes on stage for the personalized management of human lung function.Microb Biotechnol. 2024 Jun;17(6):e14506. doi: 10.1111/1751-7915.14506. Microb Biotechnol. 2024. PMID: 38881505 Free PMC article. Review.
-
Ferroptosis in periodontitis: mechanisms, impacts, and systemic connections.Cell Death Discov. 2025 Jun 20;11(1):283. doi: 10.1038/s41420-025-02550-5. Cell Death Discov. 2025. PMID: 40541947 Free PMC article. Review.
-
Connection between oral health and chronic diseases.MedComm (2020). 2025 Jan 14;6(1):e70052. doi: 10.1002/mco2.70052. eCollection 2025 Jan. MedComm (2020). 2025. PMID: 39811802 Free PMC article. Review.
-
Oral infection with periodontal pathogens induced chronic obstructive pulmonary disease-like lung changes in mice.BMC Oral Health. 2024 Jul 26;24(1):850. doi: 10.1186/s12903-024-04635-6. BMC Oral Health. 2024. PMID: 39061018 Free PMC article.
-
Periodontal disease increases the severity of chronic obstructive pulmonary disease: a Mendelian randomization study.BMC Pulm Med. 2024 May 3;24(1):220. doi: 10.1186/s12890-024-03025-6. BMC Pulm Med. 2024. PMID: 38702679 Free PMC article.
References
-
- Ahmad Hassali MA, Muhammad SA, Shah S, Abbas S, Hyder Ali IAB, Salman A. 2020. The economic burden of chronic obstructive pulmonary disease (COPD) in the USA, Europe, and Asia: results from a systematic review of the literature. Expert Rev Pharmacoecon Outcomes Res 20:661–672. doi:10.1080/14737167.2020.1678385 - DOI - PubMed
MeSH terms
Grants and funding
- 81771085/MOST | National Natural Science Foundation of China (NSFC)
- 2020YFSY0008/Key Projects of Sichuan Provincial Department of Science and Technology
- RD-02-202112/Research and Develop Program, West China Hospital of Stomatology, Sichuan University
- 2022NSFSC1507/SPDST | Natural Science Foundation of Sichuan Province ()
LinkOut - more resources
Full Text Sources
Medical
