Daptomycin avoids drug resistance mediated by the BceAB transporter in Streptococcus pneumoniae
- PMID: 38214521
- PMCID: PMC10846014
- DOI: 10.1128/spectrum.03638-23
Daptomycin avoids drug resistance mediated by the BceAB transporter in Streptococcus pneumoniae
Abstract
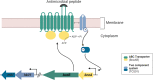
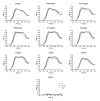
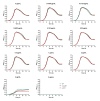
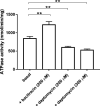
Drug-resistant bacteria are a serious threat to human health as antibiotics are gradually losing their clinical efficacy. Comprehending the mechanism of action of antimicrobials and their resistance mechanisms plays a key role in developing new agents to fight antimicrobial resistance. The lipopeptide daptomycin is an antibiotic that selectively disrupts Gram-positive bacterial membranes, thereby showing slower resistance development than many classical drugs. Consequently, it is often used as a last resort antibiotic to preserve its use as one of the least potent antibiotics at our disposal. The mode of action of daptomycin has been debated but was recently found to involve the formation of a tripartite complex between undecaprenyl precursors of cell wall biosynthesis and the anionic phospholipid phosphatidylglycerol. BceAB-type ABC transporters are known to confer resistance to antimicrobial peptides that sequester some precursors of the peptidoglycan, such as the undecaprenyl pyrophosphate or lipid II. The expression of these transporters is upregulated by dedicated two-component regulatory systems in the presence of antimicrobial peptides that are recognized by the system. Here, we investigated whether daptomycin evades resistance mediated by the BceAB transporter from the bacterial pathogen Streptococcus pneumoniae. Although daptomycin can bind to the transporter, our data showed that the BceAB transporter does not mediate resistance to the drug and its expression is not induced in its presence. These findings show that the pioneering membrane-active daptomycin has the potential to escape the resistance mechanism mediated by BceAB-type transporters and confirm that the development of this class of compounds has promising clinical applications.IMPORTANCEAntibiotic resistance is rising in all parts of the world. New resistance mechanisms are emerging and dangerously spreading, threatening our ability to treat common infectious diseases. Daptomycin is an antimicrobial peptide that is one of the last antibiotics approved for clinical use. Understanding the resistance mechanisms toward last-resort antibiotics such as daptomycin is critical for the success of future antimicrobial therapies. BceAB-type ABC transporters confer resistance to antimicrobial peptides that target precursors of cell-wall synthesis. In this study, we showed that the BceAB transporter from the human pathogen Streptococcus pneumoniae does not confer resistance to daptomycin, suggesting that this drug and other calcium-dependent lipopeptide antibiotics have the potential to evade the action of this type of ABC transporters in other bacterial pathogens.
Keywords: ABC transporters; Streptococcus pneumoniae; antibiotic resistance; antibiotics; antimicrobial peptides; two-component regulatory systems.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Identification of a two-component regulatory system involved in antimicrobial peptide resistance in Streptococcus pneumoniae.PLoS Pathog. 2022 Apr 8;18(4):e1010458. doi: 10.1371/journal.ppat.1010458. eCollection 2022 Apr. PLoS Pathog. 2022. PMID: 35395062 Free PMC article.
-
New insights into the resistance mechanism for the BceAB-type transporter SaNsrFP.Sci Rep. 2022 Mar 10;12(1):4232. doi: 10.1038/s41598-022-08095-2. Sci Rep. 2022. PMID: 35273305 Free PMC article.
-
BceAB-Type Antibiotic Resistance Transporters Appear To Act by Target Protection of Cell Wall Synthesis.Antimicrob Agents Chemother. 2020 Feb 21;64(3):e02241-19. doi: 10.1128/AAC.02241-19. Print 2020 Feb 21. Antimicrob Agents Chemother. 2020. PMID: 31871088 Free PMC article.
-
Perception and protection: The role of Bce-modules in antimicrobial peptide resistance.Biochim Biophys Acta Biomembr. 2024 Apr;1866(4):184309. doi: 10.1016/j.bbamem.2024.184309. Epub 2024 Mar 7. Biochim Biophys Acta Biomembr. 2024. PMID: 38460782 Free PMC article. Review.
-
Two-component systems regulate ABC transporters in antimicrobial peptide production, immunity and resistance.Microbiology (Reading). 2020 Jan;166(1):4-20. doi: 10.1099/mic.0.000823. Epub 2019 Jun 11. Microbiology (Reading). 2020. PMID: 31204967 Review.
Cited by
-
Guarding the walls: the multifaceted roles of Bce modules in cell envelope stress sensing and antimicrobial resistance.J Bacteriol. 2024 Jul 25;206(7):e0012324. doi: 10.1128/jb.00123-24. Epub 2024 Jun 13. J Bacteriol. 2024. PMID: 38869304 Free PMC article. Review.
-
Unlocking the power of antimicrobial peptides: advances in production, optimization, and therapeutics.Front Cell Infect Microbiol. 2025 Apr 28;15:1528583. doi: 10.3389/fcimb.2025.1528583. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40365533 Free PMC article. Review.
References
-
- Grein F, Müller A, Scherer KM, Liu X, Ludwig KC, Klöckner A, Strach M, Sahl H-G, Kubitscheck U, Schneider T. 2020. Ca(2+)-daptomycin targets cell wall biosynthesis by forming a tripartite complex with undecaprenyl-coupled intermediates and membrane lipids. Nat Commun 11:1455. doi:10.1038/s41467-020-15257-1 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

