Klebsiella pneumoniae DedA family proteins have redundant roles in divalent cation homeostasis and resistance to phagocytosis
- PMID: 38214522
- PMCID: PMC10846249
- DOI: 10.1128/spectrum.03807-23
Klebsiella pneumoniae DedA family proteins have redundant roles in divalent cation homeostasis and resistance to phagocytosis
Abstract
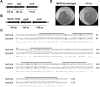
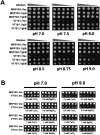
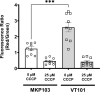
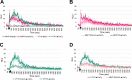
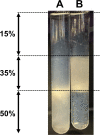
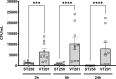
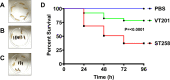
The DedA superfamily is a highly conserved family of membrane proteins. Deletion of Escherichia coli yqjA and yghB, encoding related DedA family proteins, results in sensitivity to elevated temperature, antibiotics, and alkaline pH. The human pathogen Klebsiella pneumoniae possesses genes encoding DedA family proteins with >90% amino acid identity to E. coli YqjA and YghB. We hypothesized that the deletion of K. pneumoniae yqjA and yghB will impact its physiology and may reduce its virulence. The K. pneumoniae ΔyqjA ΔyghB mutant (strain VT101) displayed a growth defect at 42°C and alkaline pH sensitivity, not unlike its E. coli counterpart. However, VT101 retained mostly wild-type resistance to antibiotics. We found VT101 was sensitive to the chelating agent EDTA, the anionic detergent SDS, and agents capable of alkalizing the bacterial cytoplasm such as bicarbonate or chloroquine. We could restore growth at alkaline pH and at elevated temperature by addition of 0.5-2 mM Ca2+ or Mg2+ to the culture media. VT101 displayed a slower uptake of calcium, which was dependent upon calcium channel activity. VT201, with similar deletions as VT101 but derived from a virulent K. pneumoniae strain, was highly susceptible to phagocytosis by alveolar macrophages and displayed a defect in the production of capsule. These findings suggest divalent cation homeostasis and virulence are interlinked by common functions of the DedA family.IMPORTANCEKlebsiella pneumoniae is a dangerous human pathogen. The DedA protein family is found in all bacteria and is a membrane transporter often required for virulence and antibiotic resistance. K. pneumoniae possesses homologs of E. coli YqjA and YghB, with 60% amino acid identity and redundant functions, which we have previously shown to be required for tolerance to biocides and alkaline pH. A K. pneumoniae strain lacking yqjA and yghB was found to be sensitive to alkaline pH, elevated temperature, and EDTA/SDS and displayed a defect in calcium uptake. Sensitivity to these conditions was reversed by addition of calcium or magnesium to the growth medium. Introduction of ΔyqjA and ΔyghB mutations into virulent K. pneumoniae resulted in the loss of capsule, increased phagocytosis by macrophages, and a partial loss of virulence. These results show that targeting the Klebsiella DedA family results in impaired divalent cation transport and, in turn, loss of virulence.
Keywords: capsular polysaccharide; divalent cations; membrane transport; phagocytosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Gorrie CL, Mirceta M, Wick RR, Edwards DJ, Thomson NR, Strugnell RA, Pratt NF, Garlick JS, Watson KM, Pilcher DV, McGloughlin SA, Spelman DW, Jenney AWJ, Holt KE. 2017. Gastrointestinal carriage is a major reservoir of Klebsiella pneumoniae infection in intensive care patients. Clin Infect Dis 65:208–215. doi: 10.1093/cid/cix270 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

