Phase 2 Trial of Cemdisiran in Adult Patients with IgA Nephropathy: A Randomized Controlled Trial
- PMID: 38214599
- PMCID: PMC11020434
- DOI: 10.2215/CJN.0000000000000384
Phase 2 Trial of Cemdisiran in Adult Patients with IgA Nephropathy: A Randomized Controlled Trial
Abstract
Background: IgA nephropathy is the most common primary GN. Clinical features of IgA nephropathy include proteinuria, which is the strongest known surrogate of progression to kidney failure. Complement pathway activation is a critical driver of inflammation and tissue injury in IgA nephropathy. Cemdisiran is an investigational RNA interference therapeutic that suppresses hepatic production of complement component 5 (C5), thereby potentially reducing proteinuria in IgA nephropathy. We evaluated the efficacy and safety of cemdisiran in adult patients with IgA nephropathy at high risk of kidney disease progression.
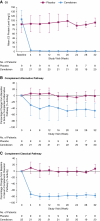
Methods: In this phase 2, 36-week, double-blind study, adult patients with IgA nephropathy and urine protein ≥1 g/24 hours were randomized (2:1) to subcutaneous cemdisiran 600 mg or placebo every 4 weeks in combination with the standard of care. The primary end point was percentage change from baseline at week 32 in urine protein-to-creatinine ratio (UPCR) measured by 24-hour urine collection. Additional end points included change from baseline in UPCR measured by spot urine, serum C5 level, and safety assessments.
Results: Thirty-one patients were randomized (cemdisiran, N =22; placebo, N =9). Cemdisiran-treated patients had a placebo-adjusted geometric mean change in 24-hour UPCR of -37.4% (cemdisiran-adjusted geometric mean ratio to baseline [SEM], 0.69 [0.10]) at week 32. Spot UPCR was consistent with 24-hour UPCR placebo-adjusted change of -45.8% (cemdisiran-adjusted geometric mean ratio to baseline [SEM], 0.73 [0.11]). Mean (SD) change in serum C5 level from baseline at week 32 was -98.7% (1.2) with cemdisiran and 25.2% (57.7) with placebo. Over 36 weeks, most adverse events were mild or moderate and transient; the most common adverse event after cemdisiran treatment was injection-site reaction (41%).
Conclusions: These findings indicate that treatment with cemdisiran resulted in a reduction of proteinuria at week 32 and was well tolerated.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Society of Nephrology.
Conflict of interest statement
P. Badri reports employment with Alnylam Pharmaceuticals Inc. and ownership interest in Alnylam Pharmaceuticals Inc., Dicerna Pharmaceuticals Inc., and Pyxis Oncology. S.J. Barbour reports consultancy for Achillion, Alexion, BeiGene, BioCryst, Chinook, Eledon, HIBio, Inception Sciences, Novartis, Pfizer, Roche, Vera, and Visterra; research funding from Alexion, Novartis, and Roche; and honoraria from Kirin. J. Barratt reports consultancy for Alebund, Alnylam Pharmaceuticals Inc., Alpine, Argenx, Astellas, BioCryst, Calliditas, Chinook, Dimerix, HiBio, Kira, Novartis, Omeros, Otsuka, Q32 Bio, Roche, Sanofi, Takeda, Travere Therapeutics, Vera Therapeutics, Vifor, and Visterra; research funding from Argenx, Calliditas, Chinook, Galapagos, GlaxoSmithKline, Novartis, Omeros, Travere Therapeutics, and Visterra; role on the Editorial Boards of
Figures




References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

