VEXAS syndrome: complete molecular remission after hypomethylating therapy
- PMID: 38214707
- PMCID: PMC10866742
- DOI: 10.1007/s00277-023-05611-w
VEXAS syndrome: complete molecular remission after hypomethylating therapy
Abstract
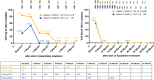
The VEXAS syndrome, a genetically defined autoimmune disease, associated with various hematological neoplasms has been attracting growing attention since its initial description in 2020. While various therapeutic strategies have been explored in case studies, the optimal treatment strategy is still under investigation and allogeneic cell transplantation is considered the only curative treatment. Here, we describe 2 patients who achieved complete molecular remission of the underlying UBA1 mutant clone outside the context of allogeneic HCT. Both patients received treatment with the hypomethylating agent azacitidine, and deep molecular remission triggered treatment de-escalation and even cessation with sustained molecular remission in one of them. Prospective studies are necessary to clarify which VEXAS patients will benefit most from hypomethylating therapy and to understand the variability in the response to different treatment strategies.
Keywords: Azacitidine; Hypomethylating therapy; Molecular remission; VEXAS.
© 2024. The Author(s).
Conflict of interest statement
KS served on advisory boards and received lecture fees from BMS/Celgene and Novartis. KSG received honoraria from BMS, Jazz Pharmaceuticals, and Abbvie. MA served on advisory boards for Novartis. MB served on advisory boards and received lecture fees from Jazz Pharmaceuticals and served on advisory boards from FarmaTrust. MB received travel compensation from DKMS. DH received research funding from BMS, Jazz Pharmaceuticals, and lecture fees from BMS/Celgene, Jazz Pharmaceuticals, and Novartis; he served on advisory boards for BMS, Novartis, Jazz Pharmaceuticals, Gilead, Takeda, and Hexal. CT is co-owner and CEO of AgenDix GmbH. CT has received lecture fees and/or participated in advisory boards from Novartis, Jazz Pharmaceuticals, Astellas, Janssen, and Illumina. CT has received research funding from Novartis and Bayer. CG, JAG, EB, and MU declare no potential conflict of interest.
Figures
References
-
- Mekinian A, Zhao LP, Chevret S et al (2022) A phase II prospective trial of azacitidine in steroid-dependent or refractory systemic autoimmune/inflammatory disorders and VEXAS syndrome associated with MDS and CMML. Leukemia 36(11):2739–2742. 10.1038/s41375-022-01698-8 10.1038/s41375-022-01698-8 - DOI - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

