Complement and MHC patterns can provide the diagnostic framework for inflammatory neuromuscular diseases
- PMID: 38214778
- PMCID: PMC10786976
- DOI: 10.1007/s00401-023-02669-8
Complement and MHC patterns can provide the diagnostic framework for inflammatory neuromuscular diseases
Abstract
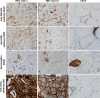
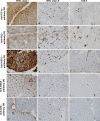
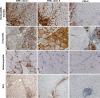
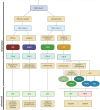
Histopathological analysis stands as the gold standard for the identification and differentiation of inflammatory neuromuscular diseases. These disorders continue to constitute a diagnostic challenge due to their clinical heterogeneity, rarity and overlapping features. To establish standardized protocols for the diagnosis of inflammatory neuromuscular diseases, the development of cost-effective and widely applicable tools is crucial, especially in settings constrained by limited resources. The focus of this review is to emphasize the diagnostic value of major histocompatibility complex (MHC) and complement patterns in the immunohistochemical analysis of these diseases. We explore the immunological background of MHC and complement signatures that characterize inflammatory features, with a specific focus on idiopathic inflammatory myopathies. With this approach, we aim to provide a diagnostic algorithm that may improve and simplify the diagnostic workup based on a limited panel of stainings. Our approach acknowledges the current limitations in the field of inflammatory neuromuscular diseases, particularly the scarcity of large-scale, prospective studies that validate the diagnostic potential of these markers. Further efforts are needed to establish a consensus on the diagnostic protocol to effectively distinguish these diseases.
Keywords: Complement; Major histocompatibility complex; Muscle dystrophies; Myasthenia gravis; Myositis.
© 2024. The Author(s).
Figures


References
-
- Allenbach Y, Anquetil C, Manouchehri A, Benveniste O, Lambotte O, Lebrun-Vignes B, Spano J-P, Ederhy S, Klatzmann D, Rosenzwajg M, Fautrel B, Cadranel J, Johnson DB, Moslehi JJ, Salem J-E. Immune checkpoint inhibitor-induced myositis, the earliest and most lethal complication among rheumatic and musculoskeletal toxicities. Autoimmun Rev. 2020;19:102586. doi: 10.1016/j.autrev.2020.102586. - DOI - PubMed
-
- Allenbach Y, Arouche-Delaperche L, Preusse C, Radbruch H, Butler-Browne G, Champtiaux N, Mariampillai K, Rigolet A, Hufnagl P, Zerbe N, Amelin D, Maisonobe T, Louis-Leonard S, Duyckaerts C, Eymard B, Goebel H-H, Bergua C, Drouot L, Boyer O, Benveniste O, Stenzel W. Necrosis in anti-SRP+ and anti-HMGCR+myopathies: role of autoantibodies and complement. Neurology. 2018;90:e507–e517. doi: 10.1212/WNL.0000000000004923. - DOI - PubMed
-
- Allenbach Y, Leroux G, Suárez-Calvet X, Preusse C, Gallardo E, Hervier B, Rigolet A, Hie M, Pehl D, Limal N, Hufnagl P, Zerbe N, Meyer A, Aouizerate J, Uzunhan Y, Maisonobe T, Goebel H-H, Benveniste O, Stenzel W, Network FM. Dermatomyositis with or without anti-melanoma differentiation-associated gene 5 antibodies: common interferon signature but distinct NOS2 expression. Am J Pathol. 2016;186:691–700. doi: 10.1016/j.ajpath.2015.11.010. - DOI - PubMed
-
- Allenbach Y, Mammen AL, Benveniste O, Stenzel W, Immune-Mediated Necrotizing Myopathies Working Group 224th ENMC international workshop: clinico-sero-pathological classification of immune-mediated necrotizing myopathies Zandvoort, The Netherlands, 14–16 October 2016. Neuromuscul Disord. 2018;28:87–99. doi: 10.1016/j.nmd.2017.09.016. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

