Sleep disturbance and sympathetic neural reactivity in postmenopausal females
- PMID: 38214902
- PMCID: PMC11221801
- DOI: 10.1152/ajpheart.00724.2023
Sleep disturbance and sympathetic neural reactivity in postmenopausal females
Abstract
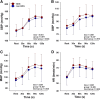
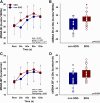
Sleep disturbance, one of the most common menopausal symptoms, contributes to autonomic dysfunction and is linked to hypertension and cardiovascular risk. Longitudinal studies suggest that hyperreactivity of blood pressure (BP) to a stressor can predict the future development of hypertension. It remains unknown if postmenopausal females who experience sleep disturbance (SDG) demonstrate greater hemodynamic and sympathetic neural hyperreactivity to a stressor. We hypothesized that postmenopausal females with reported sleep disturbance would exhibit increased hemodynamic and sympathetic reactivity to a stressor compared with postmenopausal females without sleep disturbance (non-SDG). Fifty-five postmenopausal females (age, 62 ± 4 yr old; SDG, n = 36; non-SDG; n = 19) completed two study visits. The Menopause-Specific Quality of Life Questionnaire (MENQOL) was used to assess the presence of sleep disturbance (MENQOL sleep scale, ≥2 units). Beat-to-beat BP (finger plethysmography), heart rate (HR; electrocardiogram), and muscle sympathetic nerve activity (MSNA; microneurography; SDG, n = 25; non-SDG, n = 15) were continuously measured during a 10-min baseline and 2-min stressor (cold pressor test; CPT) in both groups. Menopause age and body mass index were similar between groups (P > 0.05). There were no differences between resting BP, HR, or MSNA (P > 0.05). HR and BP reactivity were not different between SDG and non-SDG (P > 0.05). In contrast, MSNA reactivity had a more rapid increase in the first 30 s of the CPT in the SDG (burst incidence, Δ10.2 ± 14.8 bursts/100 hb) compared with the non-SDG (burst incidence, Δ4.0 ± 14.8 bursts/100 hb, time × group, P = 0.011). Our results demonstrate a more rapid sympathetic neural reactivity to a CPT in postmenopausal females with perceived sleep disturbance, a finding that aligns with and advances recent evidence that sleep disturbance is associated with sympathetic neural hyperactivity in postmenopausal females.NEW & NOTEWORTHY This is the first study to demonstrate that muscle sympathetic nerve activity (MSNA) to a cold pressor test is augmented in postmenopausal females with perceived sleep disturbance. The more rapid increase in MSNA reactivity during the cold pressor test in the sleep disturbance group was present despite similar increases in the perceived pain levels between groups. Baseline MSNA burst incidence and burst frequency, as well as blood pressure and heart rate, were similar between the sleep disturbance and nonsleep disturbance groups.
Keywords: autonomic nervous system; cardiovascular risk; menopause symptoms; microneurography; women’s health.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Jason Carter is an editor of
Figures

Similar articles
-
Sympathetic transduction at rest and during cold pressor test in young healthy non-Hispanic Black and White women.Am J Physiol Regul Integr Comp Physiol. 2023 Dec 1;325(6):R682-R691. doi: 10.1152/ajpregu.00073.2023. Epub 2023 Oct 2. Am J Physiol Regul Integr Comp Physiol. 2023. PMID: 37781734 Free PMC article.
-
Impact of age and sex on neural cardiovascular responsiveness to cold pressor test in humans.Am J Physiol Regul Integr Comp Physiol. 2020 Sep 1;319(3):R288-R295. doi: 10.1152/ajpregu.00045.2020. Epub 2020 Jul 22. Am J Physiol Regul Integr Comp Physiol. 2020. PMID: 32697654 Free PMC article.
-
Actigraphy-based sleep and muscle sympathetic nerve activity in humans.Am J Physiol Regul Integr Comp Physiol. 2024 Aug 1;327(2):R145-R151. doi: 10.1152/ajpregu.00113.2024. Epub 2024 Jun 6. Am J Physiol Regul Integr Comp Physiol. 2024. PMID: 38842513 Free PMC article.
-
Influence of Obstructive Sleep Apnea Severity on Muscle Sympathetic Nerve Activity and Blood Pressure: a Systematic Review and Meta-Analysis.Hypertension. 2022 Sep;79(9):2091-2104. doi: 10.1161/HYPERTENSIONAHA.122.19288. Epub 2022 Jun 29. Hypertension. 2022. PMID: 35766054
-
The impact of exercise training on muscle sympathetic nerve activity: a systematic review and meta-analysis.J Appl Physiol (1985). 2024 Aug 1;137(2):429-444. doi: 10.1152/japplphysiol.00060.2024. Epub 2024 May 16. J Appl Physiol (1985). 2024. PMID: 38752285
Cited by
-
Menopause and its effects on autonomic regulation of blood pressure: Insights and perspectives.Auton Neurosci. 2025 Aug;260:103295. doi: 10.1016/j.autneu.2025.103295. Epub 2025 May 29. Auton Neurosci. 2025. PMID: 40460599 Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

