Transcriptome and small RNAome profiling uncovers how a recombinant begomovirus evades RDRγ-mediated silencing of viral genes and outcompetes its parental virus in mixed infection
- PMID: 38215155
- PMCID: PMC10810479
- DOI: 10.1371/journal.ppat.1011941
Transcriptome and small RNAome profiling uncovers how a recombinant begomovirus evades RDRγ-mediated silencing of viral genes and outcompetes its parental virus in mixed infection
Abstract
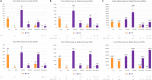
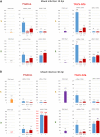
Tomato yellow leaf curl virus (TYLCV, genus Begomovirus, family Geminiviridae) causes severe disease of cultivated tomatoes. Geminiviruses replicate circular single-stranded genomic DNA via rolling-circle and recombination-dependent mechanisms, frequently generating recombinants in mixed infections. Circular double-stranded intermediates of replication also serve as templates for Pol II bidirectional transcription. IS76, a recombinant derivative of TYLCV with a short sequence in the bidirectional promoter/origin-of-replication region acquired from a related begomovirus, outcompetes TYLCV in mixed infection and breaks disease resistance in tomato Ty-1 cultivars. Ty-1 encodes a γ-clade RNA-dependent RNA polymerase (RDRγ) implicated in Dicer-like (DCL)-mediated biogenesis of small interfering (si)RNAs directing gene silencing. Here, we profiled transcriptome and small RNAome of Ty-1 resistant and control susceptible plants infected with TYLCV, IS76 or their combination at early and late infection stages. We found that RDRγ boosts production rates of 21, 22 and 24 nt siRNAs from entire genomes of both viruses and modulates DCL activities in favour of 22 and 24 nt siRNAs. Compared to parental TYLCV, IS76 undergoes faster transition to the infection stage favouring rightward transcription of silencing suppressor and coat protein genes, thereby evading RDRγ activity and facilitating its DNA accumulation in both single and mixed infections. In coinfected Ty-1 plants, IS76 efficiently competes for host replication and transcription machineries, thereby impairing TYLCV replication and transcription and forcing its elimination associated with further increased siRNA production. RDRγ is constitutively overexpressed in Ty-1 plants, which correlates with begomovirus resistance, while siRNA-generating DCLs (DCL2b/d, DCL3, DCL4) and genes implicated in siRNA amplification (α-clade RDR1) and function (Argonaute2) are upregulated to similar levels in TYLCV- and IS76-infected susceptible plants. Collectively, IS76 recombination facilitates replication and promotes expression of silencing suppressor and coat proteins, which allows the recombinant virus to evade the negative impact of RDRγ-boosted production of viral siRNAs directing transcriptional and posttranscriptional silencing.
Copyright: © 2024 Jammes et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors declare no competing interests.
Figures










References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

