Development of Polarity-Reversed Endometrial Epithelial Organoids
- PMID: 38215284
- PMCID: PMC10959009
- DOI: 10.1530/REP-23-0478
Development of Polarity-Reversed Endometrial Epithelial Organoids
Abstract
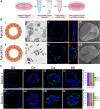
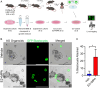
The uterine epithelium is composed of a single layer of hormone responsive polarized epithelial cells that line the lumen and form tubular glands. Endometrial epithelial organoids (EEO) can be generated from uterine epithelia and recapitulate cell composition and hormone responses in vitro. As such, the development of EEO represents a major advance for facilitating mechanistic studies in vitro. However, a major limitation for the use of EEO cultured in basement membrane extract and other hydrogels is the inner location of apical membrane, thereby hindering direct access to the apical surface of the epithelium to study interactions with the embryo or infectious agents such as viruses and bacteria. Here, a straightforward strategy was developed that successfully reverses the polarity of EEO. The result is an apical-out organoid that preserves a distinct apical-basolateral orientation and remains responsive to ovarian steroid hormones. Our investigations highlight the utility of polarity-reversed EEO to study interactions with E. coli and blastocysts. This method of generating apical-out EEO lays the foundation for developing new in vitro functional assays, particularly regarding epithelial interactions with embryos during pregnancy or other luminal constituents in a pathological or diseased state.
Conflict of interest statement
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Figures


Update of
-
Development of Polarity-Reversed Endometrial Epithelial Organoids.bioRxiv [Preprint]. 2023 Aug 19:2023.08.18.553918. doi: 10.1101/2023.08.18.553918. bioRxiv. 2023. Update in: Reproduction. 2024 Jan 1:REP-23-0478. doi: 10.1530/REP-23-0478. PMID: 37645779 Free PMC article. Updated. Preprint.
References
-
- Boggavarapu NR Lalitkumar S Joshua V Kasvandik S Salumets A Lalitkumar PG & Gemzell-Danielsson K. 2016Compartmentalized gene expression profiling of receptive endometrium reveals progesterone regulated ENPP3 is differentially expressed and secreted in glycosylated form. Scientific Reports 633811. (10.1038/srep33811) - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

