Fludarabine, Cytarabine, Granulocyte Colony-Stimulating Factor, and Idarubicin With Gemtuzumab Ozogamicin Improves Event-Free Survival in Younger Patients With Newly Diagnosed AML and Overall Survival in Patients With NPM1 and FLT3 Mutations
- PMID: 38215358
- PMCID: PMC11003505
- DOI: 10.1200/JCO.23.00943
Fludarabine, Cytarabine, Granulocyte Colony-Stimulating Factor, and Idarubicin With Gemtuzumab Ozogamicin Improves Event-Free Survival in Younger Patients With Newly Diagnosed AML and Overall Survival in Patients With NPM1 and FLT3 Mutations
Abstract
Purpose: To determine the optimal induction chemotherapy regimen for younger adults with newly diagnosed AML without known adverse risk cytogenetics.
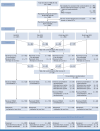
Patients and methods: One thousand thirty-three patients were randomly assigned to intensified (fludarabine, cytarabine, granulocyte colony-stimulating factor, and idarubicin [FLAG-Ida]) or standard (daunorubicin and Ara-C [DA]) induction chemotherapy, with one or two doses of gemtuzumab ozogamicin (GO). The primary end point was overall survival (OS).
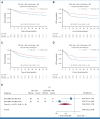
Results: There was no difference in remission rate after two courses between FLAG-Ida + GO and DA + GO (complete remission [CR] + CR with incomplete hematologic recovery 93% v 91%) or in day 60 mortality (4.3% v 4.6%). There was no difference in OS (66% v 63%; P = .41); however, the risk of relapse was lower with FLAG-Ida + GO (24% v 41%; P < .001) and 3-year event-free survival was higher (57% v 45%; P < .001). In patients with an NPM1 mutation (30%), 3-year OS was significantly higher with FLAG-Ida + GO (82% v 64%; P = .005). NPM1 measurable residual disease (MRD) clearance was also greater, with 88% versus 77% becoming MRD-negative in peripheral blood after cycle 2 (P = .02). Three-year OS was also higher in patients with a FLT3 mutation (64% v 54%; P = .047). Fewer transplants were performed in patients receiving FLAG-Ida + GO (238 v 278; P = .02). There was no difference in outcome according to the number of GO doses, although NPM1 MRD clearance was higher with two doses in the DA arm. Patients with core binding factor AML treated with DA and one dose of GO had a 3-year OS of 96% with no survival benefit from FLAG-Ida + GO.
Conclusion: Overall, FLAG-Ida + GO significantly reduced relapse without improving OS. However, exploratory analyses show that patients with NPM1 and FLT3 mutations had substantial improvements in OS. By contrast, in patients with core binding factor AML, outcomes were excellent with DA + GO with no FLAG-Ida benefit.
Trial registration: ClinicalTrials.gov ISRCTN78449203.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures


References
-
- Döhner H, Wei AH, Appelbaum FR, et al. : Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood 140:1345-1377, 2022 - PubMed
-
- Burnett AK, Russell NH, Hills RK, et al. : Optimization of chemotherapy for younger patients with acute myeloid leukemia: Results of the medical research council AML15 trial. J Clin Oncol 31:3360-3368, 2013 - PubMed
-
- Burnett AK, Hills RK, Milligan D, et al. : Identification of patients with acute myeloblastic leukemia who benefit from the addition of gemtuzumab ozogamicin: Results of the MRC AML15 trial. J Clin Oncol 29:369-377, 2011 - PubMed
-
- Castaigne S, Pautas C, Terré C, et al. : Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): A randomised, open-label, phase 3 study. Lancet 379:1508-1516, 2012 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

