Neural circuit disruptions of eye gaze processing in autism spectrum disorder and schizophrenia: An activation likelihood estimation meta-analysis
- PMID: 38215566
- PMCID: PMC10922721
- DOI: 10.1016/j.schres.2023.12.003
Neural circuit disruptions of eye gaze processing in autism spectrum disorder and schizophrenia: An activation likelihood estimation meta-analysis
Abstract
Background: Impairment in social cognition, particularly eye gaze processing, is a shared feature common to autism spectrum disorder (ASD) and schizophrenia. However, it is unclear if a convergent neural mechanism also underlies gaze dysfunction in these conditions. The present study examined whether this shared eye gaze phenotype is reflected in a profile of convergent neurobiological dysfunction in ASD and schizophrenia.
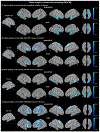
Methods: Activation likelihood estimation (ALE) meta-analyses were conducted on peak voxel coordinates across the whole brain to identify spatial convergence. Functional coactivation with regions emerging as significant was assessed using meta-analytic connectivity modeling. Functional decoding was also conducted.
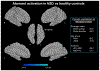
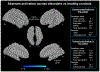
Results: Fifty-six experiments (n = 30 with schizophrenia and n = 26 with ASD) from 36 articles met inclusion criteria, which comprised 354 participants with ASD, 275 with schizophrenia and 613 healthy controls (1242 participants in total). In ASD, aberrant activation was found in the left amygdala relative to unaffected controls during gaze processing. In schizophrenia, aberrant activation was found in the right inferior frontal gyrus and supplementary motor area. Across ASD and schizophrenia, aberrant activation was found in the right inferior frontal gyrus and right fusiform gyrus during gaze processing. Functional decoding mapped the left amygdala to domains related to emotion processing and cognition, the right inferior frontal gyrus to cognition and perception, and the right fusiform gyrus to visual perception, spatial cognition, and emotion perception. These regions also showed meta-analytic connectivity to frontoparietal and frontotemporal circuitry.
Conclusion: Alterations in frontoparietal and frontotemporal circuitry emerged as neural markers of gaze impairments in ASD and schizophrenia. These findings have implications for advancing transdiagnostic biomarkers to inform targeted treatments for ASD and schizophrenia.
Keywords: Amygdala; Autism spectrum disorder; Cognitive control; Eye gaze; Lateral prefrontal cortex; Schizophrenia.
Copyright © 2023 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare no competing interests.
Figures



Similar articles
-
Meta-analytic connectivity modelling of functional magnetic resonance imaging studies in autism spectrum disorders.Brain Imaging Behav. 2023 Apr;17(2):257-269. doi: 10.1007/s11682-022-00754-2. Epub 2023 Jan 12. Brain Imaging Behav. 2023. PMID: 36633738 Free PMC article. Review.
-
Speech perception in autism spectrum disorder: An activation likelihood estimation meta-analysis.Behav Brain Res. 2018 Feb 15;338:118-127. doi: 10.1016/j.bbr.2017.10.025. Epub 2017 Oct 23. Behav Brain Res. 2018. PMID: 29074403
-
Frontoparietal Network in Executive Functioning in Autism Spectrum Disorder.Autism Res. 2020 Oct;13(10):1762-1777. doi: 10.1002/aur.2403. Epub 2020 Oct 5. Autism Res. 2020. PMID: 33016005
-
Hemispheric differences in language processing in autism spectrum disorders: A meta-analysis of neuroimaging studies.Autism Res. 2016 Oct;9(10):1046-1057. doi: 10.1002/aur.1599. Epub 2016 Jan 11. Autism Res. 2016. PMID: 26751141
-
[Social cognition in schizophrenia and autism spectrum disorder: Points of convergence and functional differences].Encephale. 2018 Dec;44(6):523-537. doi: 10.1016/j.encep.2018.03.004. Epub 2018 Aug 16. Encephale. 2018. PMID: 30122298 Review. French.
Cited by
-
An Honest Reckoning With the Amygdala and Mental Illness.Am J Psychiatry. 2024 Dec 1;181(12):1059-1075. doi: 10.1176/appi.ajp.20240941. Am J Psychiatry. 2024. PMID: 39616453 Free PMC article. Review.
References
-
- Adams RB Jr., Gordon HL, Baird AA, Ambady N, Kleck RE, 2003. Effects of gaze on amygdala sensitivity to anger and fear faces. Science (New York, N.Y.) 300(5625), 1536. - PubMed
-
- American Psychiatric Association, 2013. Diagnostic and statistical manual of mental disorders, 5th ed. Author, Washington, DC.
-
- Amodio DM, Frith CD, 2006. Meeting of minds: the medial frontal cortex and social cognition. Nature reviews. Neuroscience 7(4), 268–277. - PubMed
-
- Aron AR, Robbins TW, Poldrack RA, 2014. Inhibition and the right inferior frontal cortex: one decade on. Trends in cognitive sciences 18(4), 177–185. - PubMed
-
- Baas D, Van’t Wout M, Aleman A, Kahn R, 2008. Social judgement in clinically stable patients with schizophrenia and healthy relatives: behavioural evidence of social brain dysfunction. Psychological medicine 38(5), 747–754. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

