The interaction between NLRP1 and oxidized TRX1 involves a transient disulfide bond
- PMID: 38215746
- PMCID: PMC11102328
- DOI: 10.1016/j.chembiol.2023.12.012
The interaction between NLRP1 and oxidized TRX1 involves a transient disulfide bond
Abstract
NLRP1 is an innate immune receptor that detects pathogen-associated signals, assembles into a multiprotein structure called an inflammasome, and triggers a proinflammatory form of cell death called pyroptosis. We previously discovered that the oxidized, but not the reduced, form of thioredoxin-1 directly binds to NLRP1 and represses inflammasome formation. However, the molecular basis for NLRP1's selective association with only the oxidized form of TRX1 has not yet been established. Here, we leveraged AlphaFold-Multimer, site-directed mutagenesis, thiol-trapping experiments, and mass spectrometry to reveal that a specific cysteine residue (C427 in humans) on NLRP1 forms a transient disulfide bond with oxidized TRX1. Overall, this work demonstrates how NLRP1 monitors the cellular redox state, further illuminating an unexpected connection between the intracellular redox potential and the innate immune system.
Keywords: AlphaFold-Multimer; NLRP1; disulfide; inflammasome; pyroptosis; redox; thioredoxin.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures
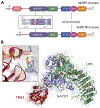


Update of
-
The interaction between NLRP1 and oxidized TRX1 involves a transient disulfide bond.bioRxiv [Preprint]. 2023 Sep 28:2023.09.27.559829. doi: 10.1101/2023.09.27.559829. bioRxiv. 2023. Update in: Cell Chem Biol. 2024 May 16;31(5):955-961.e4. doi: 10.1016/j.chembiol.2023.12.012. PMID: 37808697 Free PMC article. Updated. Preprint.
Similar articles
-
The interaction between NLRP1 and oxidized TRX1 involves a transient disulfide bond.bioRxiv [Preprint]. 2023 Sep 28:2023.09.27.559829. doi: 10.1101/2023.09.27.559829. bioRxiv. 2023. Update in: Cell Chem Biol. 2024 May 16;31(5):955-961.e4. doi: 10.1016/j.chembiol.2023.12.012. PMID: 37808697 Free PMC article. Updated. Preprint.
-
Oxidized thioredoxin-1 restrains the NLRP1 inflammasome.Sci Immunol. 2022 Nov 11;7(77):eabm7200. doi: 10.1126/sciimmunol.abm7200. Epub 2022 Nov 4. Sci Immunol. 2022. PMID: 36332009 Free PMC article.
-
Structural basis for thioredoxin-mediated suppression of NLRP1 inflammasome.Nature. 2023 Oct;622(7981):188-194. doi: 10.1038/s41586-023-06532-4. Epub 2023 Sep 13. Nature. 2023. PMID: 37704723
-
The NLRP1 and CARD8 inflammasomes.Immunol Rev. 2020 Sep;297(1):13-25. doi: 10.1111/imr.12884. Epub 2020 Jun 19. Immunol Rev. 2020. PMID: 32558991 Free PMC article. Review.
-
The NLRP1 inflammasomes.Immunol Rev. 2015 May;265(1):22-34. doi: 10.1111/imr.12283. Immunol Rev. 2015. PMID: 25879281 Review.
Cited by
-
The serine protease DPP9 and the redox sensor KEAP1 form a mutually inhibitory complex.J Biol Chem. 2025 Jan;301(1):108034. doi: 10.1016/j.jbc.2024.108034. Epub 2024 Nov 29. J Biol Chem. 2025. PMID: 39615677 Free PMC article.
-
The hydrophobicity of the CARD8 N-terminus tunes inflammasome activation.Cell Chem Biol. 2024 Sep 19;31(9):1699-1713.e8. doi: 10.1016/j.chembiol.2024.06.004. Epub 2024 Jul 10. Cell Chem Biol. 2024. PMID: 38991619
References
-
- Martinon F, Burns K, and Tschopp J (2002). The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell 10, 417–426. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

