Response to Bruton's tyrosine kinase inhibitors in aggressive lymphomas linked to chronic selective autophagy
- PMID: 38215749
- PMCID: PMC11256978
- DOI: 10.1016/j.ccell.2023.12.019
Response to Bruton's tyrosine kinase inhibitors in aggressive lymphomas linked to chronic selective autophagy
Abstract
Diffuse large B cell lymphoma (DLBCL) is an aggressive, profoundly heterogeneous cancer, presenting a challenge for precision medicine. Bruton's tyrosine kinase (BTK) inhibitors block B cell receptor (BCR) signaling and are particularly effective in certain molecular subtypes of DLBCL that rely on chronic active BCR signaling to promote oncogenic NF-κB. The MCD genetic subtype, which often acquires mutations in the BCR subunit, CD79B, and in the innate immune adapter, MYD88L265P, typically resists chemotherapy but responds exceptionally to BTK inhibitors. However, the underlying mechanisms of response to BTK inhibitors are poorly understood. Herein, we find a non-canonical form of chronic selective autophagy in MCD DLBCL that targets ubiquitinated MYD88L265P for degradation in a TBK1-dependent manner. MCD tumors acquire genetic and epigenetic alterations that attenuate this autophagic tumor suppressive pathway. In contrast, BTK inhibitors promote autophagic degradation of MYD88L265P, thus explaining their exceptional clinical benefit in MCD DLBCL.
Keywords: Bruton’s tyrosine kinase; DLBCL; autophagy; functional genomics; proteomics; targeted therapy.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests T.O. received research funding from Gilead and Merck KGaA, is a consultant/received honoraria for/from Beigene, Roche, Janssen, Merck KGaA, Gilead, Kronos Bio and Abbvie (all not related to this work). Z.W. is a current employee at GSK. A.L.S III is a current employee at AstraZeneca and has stock options.
Figures

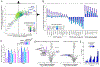

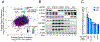


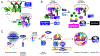
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

