A bacterial vesicle-based pneumococcal vaccine against influenza-mediated secondary Streptococcus pneumoniae pulmonary infection
- PMID: 38215909
- PMCID: PMC11033695
- DOI: 10.1016/j.mucimm.2024.01.002
A bacterial vesicle-based pneumococcal vaccine against influenza-mediated secondary Streptococcus pneumoniae pulmonary infection
Abstract
Streptococcus pneumoniae (Spn) is a common pathogen causing a secondary bacterial infection following influenza, which leads to severe morbidity and mortality during seasonal and pandemic influenza. Therefore, there is an urgent need to develop bacterial vaccines that prevent severe post-influenza bacterial pneumonia. Here, an improved Yersinia pseudotuberculosis strain (designated as YptbS46) possessing an Asd+ plasmid pSMV92 could synthesize high amounts of the Spn pneumococcal surface protein A (PspA) antigen and monophosphoryl lipid A as an adjuvant. The recombinant strain produced outer membrane vesicles (OMVs) enclosing a high amount of PspA protein (designated as OMV-PspA). A prime-boost intramuscular immunization with OMV-PspA induced both memory adaptive and innate immune responses in vaccinated mice, reduced the viral and bacterial burden, and provided complete protection against influenza-mediated secondary Spn infection. Also, the OMV-PspA immunization afforded significant cross-protection against the secondary Spn A66.1 infection and long-term protection against the secondary Spn D39 challenge. Our study implies that an OMV vaccine delivering Spn antigens can be a new promising pneumococcal vaccine candidate.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
DECLARATIONS OF COMPETING INTEREST
The authors have no competing interests to declare.
Figures
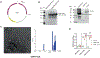
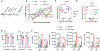


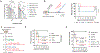

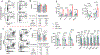
References
-
- Centers for Disease Control and Prevention. Pneumococcal disease. Available at: https://www.cdc.gov/pneumococcal/index.html [Date accessed: 15 June 2019].
-
- Sun K & Metzger DW Inhibition of pulmonary antibacterial defense by interferon-gamma during recovery from influenza infection. Nat. Med 14, 558–564 (2008). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

