Single-cell multi-omic analysis of the vestibular schwannoma ecosystem uncovers a nerve injury-like state
- PMID: 38216553
- PMCID: PMC10786875
- DOI: 10.1038/s41467-023-42762-w
Single-cell multi-omic analysis of the vestibular schwannoma ecosystem uncovers a nerve injury-like state
Abstract
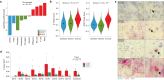
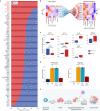
Vestibular schwannomas (VS) are benign tumors that lead to significant neurologic and otologic morbidity. How VS heterogeneity and the tumor microenvironment (TME) contribute to VS pathogenesis remains poorly understood. In this study, we perform scRNA-seq on 15 VS, with paired scATAC-seq (n = 6) and exome sequencing (n = 12). We identify diverse Schwann cell (SC), stromal, and immune populations in the VS TME and find that repair-like and MHC-II antigen-presenting SCs are associated with myeloid cell infiltrate, implicating a nerve injury-like process. Deconvolution analysis of RNA-expression data from 175 tumors reveals Injury-like tumors are associated with larger tumor size, and scATAC-seq identifies transcription factors associated with nerve repair SCs from Injury-like tumors. Ligand-receptor analysis and in vitro experiments suggest that Injury-like VS-SCs recruit myeloid cells via CSF1 signaling. Our study indicates that Injury-like SCs may cause tumor growth via myeloid cell recruitment and identifies molecular pathways that may be therapeutically targeted.
© 2023. The Author(s).
Conflict of interest statement
Regarding potential conflicts of interest, A.H.K. is a consultant for Monteris Medical and has received non-related research grants from Stryker and Collagen Matrix for study of a dural substitute. C.C.W. is a consultant for Stryker and Cochlear Ltd. C.A.B. is a consultant for Advanced Bionics, Cochlear, Envoy, and IotaMotion, and also has equity interest in Advanced Cochlear Diagnostics L.L.C. The remaining authors declare no competing interests.
Figures



References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

