Assessment of safety profile of secukinumab in real-world scenario using United States food and drug administration adverse event reporting system database
- PMID: 38216608
- PMCID: PMC10786882
- DOI: 10.1038/s41598-023-50013-7
Assessment of safety profile of secukinumab in real-world scenario using United States food and drug administration adverse event reporting system database
Abstract
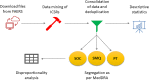
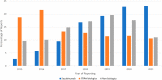
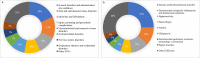
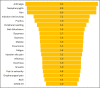
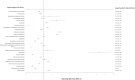
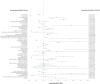
Secukinumab is an anti-IL-17 monoclonal antibody approved for treating psoriasis and various arthritides. A comprehensive evaluation of its safety, especially in a real-world setting, is necessary. This study aimed to describe the adverse events (AE) associated with secukinumab use using the United States Food and Drug Administration Adverse Event Reporting System (FAERS) database. FAERS data files containing AE reports from 2015 to 2021 were downloaded for data mining. Primary or secondary suspect medications indicated for psoriasis were identified and analyzed. Medical dictionary for regulatory activities (MedDRA version 24.1) was used to analyze the AE terms. To detect potential safety signals of AE from secukinumab use, disproportionality analysis was used. A total of 365,590 adverse event reports were identified; of these, 44,761 reports involved the use of secukinumab. Safety signals were identified for ocular infections and gastrointestinal adverse events at the standardised MedDRA query level. Safety signals for oral candidiasis, oral herpes, conjunctivitis, eye infections, and ulcerative colitis were identified at the preferred term level. The findings of our study are consistent with those of earlier studies, such as the increased risk of infections and inflammatory bowel disease. However, our study also identified additional safety signals that need to be further evaluated.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Toxicity signals associated with secukinumab: A pharmacovigilance study based on the United States Food and Drug Administration Adverse Event Reporting System database.Br J Clin Pharmacol. 2023 Feb;89(2):865-873. doi: 10.1111/bcp.15535. Epub 2022 Oct 7. Br J Clin Pharmacol. 2023. PMID: 36106653
-
Data mining and analysis of adverse event signals associated with teprotumumab using the Food and Drug Administration adverse event reporting system database.Int J Clin Pharm. 2024 Apr;46(2):471-479. doi: 10.1007/s11096-023-01676-9. Epub 2024 Jan 20. Int J Clin Pharm. 2024. PMID: 38245664
-
Potential cerebrovascular accident signal for risankizumab: A disproportionality analysis of the FDA Adverse Event Reporting System (FAERS).Br J Clin Pharmacol. 2023 Aug;89(8):2386-2395. doi: 10.1111/bcp.15581. Epub 2022 Nov 15. Br J Clin Pharmacol. 2023. PMID: 36321844
-
Analysis of Spontaneous Postmarket Case Reports Submitted to the FDA Regarding Thromboembolic Adverse Events and JAK Inhibitors.Drug Saf. 2018 Apr;41(4):357-361. doi: 10.1007/s40264-017-0622-2. Drug Saf. 2018. PMID: 29196988 Review.
-
Safety profile of clobazam in the real world: an analysis of FAERS database and systematic review of case reports.Expert Opin Drug Saf. 2024 Jan;23(1):119-128. doi: 10.1080/14740338.2023.2204227. Epub 2023 Apr 19. Expert Opin Drug Saf. 2024. PMID: 37070461
Cited by
-
Adverse events of topical ocular prostaglandin medications for glaucoma treatment: a pharmacovigilance study based on the FAERS database.Ther Adv Drug Saf. 2024 Oct 16;15:20420986241285929. doi: 10.1177/20420986241285929. eCollection 2024. Ther Adv Drug Saf. 2024. PMID: 39429679 Free PMC article.
-
Evaluate the renal system damage caused by zoledronic acid: a comprehensive analysis of adverse events from FAERS.BMC Cancer. 2024 Dec 18;24(1):1520. doi: 10.1186/s12885-024-13284-5. BMC Cancer. 2024. PMID: 39695477 Free PMC article.
-
Targeting Common Inflammatory Mediators in Experimental Severe Asthma and Acute Lung Injury.Pharmaceuticals (Basel). 2024 Mar 5;17(3):338. doi: 10.3390/ph17030338. Pharmaceuticals (Basel). 2024. PMID: 38543124 Free PMC article.
-
Secukinumab in the Treatment of Psoriasis: A Narrative Review on Early Treatment and Real-World Evidence.Dermatol Ther (Heidelb). 2024 Oct;14(10):2739-2757. doi: 10.1007/s13555-024-01255-4. Epub 2024 Sep 24. Dermatol Ther (Heidelb). 2024. PMID: 39316358 Free PMC article. Review.
-
Exploratory disproportionality analysis of potentially drug-induced eosinophilic pneumonia using United States Food and Drug Administration adverse event reporting system.Sci Rep. 2025 Jan 9;15(1):1455. doi: 10.1038/s41598-025-85681-0. Sci Rep. 2025. PMID: 39789288 Free PMC article.
References
-
- Biological Product Definitions | FDA. https://www.fda.gov/files/drugs/published/Biological-Product-Definitions....
-
- What Are ‘Biologics’ Questions and Answers | FDA. https://www.fda.gov/about-fda/center-biologics-evaluation-and-research-c....
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

