Basal MET phosphorylation is an indicator of hepatocyte dysregulation in liver disease
- PMID: 38216754
- PMCID: PMC10912216
- DOI: 10.1038/s44320-023-00007-4
Basal MET phosphorylation is an indicator of hepatocyte dysregulation in liver disease
Abstract
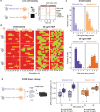
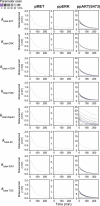
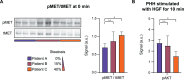
Chronic liver diseases are worldwide on the rise. Due to the rapidly increasing incidence, in particular in Western countries, metabolic dysfunction-associated steatotic liver disease (MASLD) is gaining importance as the disease can develop into hepatocellular carcinoma. Lipid accumulation in hepatocytes has been identified as the characteristic structural change in MASLD development, but molecular mechanisms responsible for disease progression remained unresolved. Here, we uncover in primary hepatocytes from a preclinical model fed with a Western diet (WD) an increased basal MET phosphorylation and a strong downregulation of the PI3K-AKT pathway. Dynamic pathway modeling of hepatocyte growth factor (HGF) signal transduction combined with global proteomics identifies that an elevated basal MET phosphorylation rate is the main driver of altered signaling leading to increased proliferation of WD-hepatocytes. Model-adaptation to patient-derived hepatocytes reveal patient-specific variability in basal MET phosphorylation, which correlates with patient outcome after liver surgery. Thus, dysregulated basal MET phosphorylation could be an indicator for the health status of the liver and thereby inform on the risk of a patient to suffer from liver failure after surgery.
Keywords: Dynamic Pathway Modeling; Fatty Liver Disease; HGF Signal Transduction; Hepatocyte Dysregulation; Western Diet.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests. UK is an editorial advisory board member. This has no bearing on the editorial consideration of this article for publication.
Figures









References
-
- Afgan E, Baker D, van den Beek M, Blankenberg D, Bouvier D, Cech M, Chilton J, Clements D, Coraor N, Eberhard C, et al. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2016 update. Nucleic Acids Res. 2016;44:W3–W10. doi: 10.1093/nar/gkw343. - DOI - PMC - PubMed
-
- Allaire M, Gilgenkrantz H (2018) The impact of steatosis on liver regeneration. Horm Mol Biol Clin Investig. 10.1515/hmbci-2018-0050 - PubMed
-
- Best DJ, Roberts DE. Algorithm AS 89: the upper tail probabilities of Spearman’s rho. J R Stat Soc Ser C (Appl Stat) 1975;24:377–379.
MeSH terms
Substances
Grants and funding
- 031L0042/Bundesministerium für Bildung und Forschung (BMBF)
- 031L0048/Bundesministerium für Bildung und Forschung (BMBF)
- 031L0256A/Bundesministerium für Bildung und Forschung (BMBF)
- 031L0257C/Bundesministerium für Bildung und Forschung (BMBF)
- 031L0212B/Bundesministerium für Bildung und Forschung (BMBF)
- 82DZL004A4/Bundesministerium für Bildung und Forschung (BMBF)
- 031L0256G/Bundesministerium für Bildung und Forschung (BMBF)
- 031L0257K/Bundesministerium für Bildung und Forschung (BMBF)
- 031L0045/Bundesministerium für Bildung und Forschung (BMBF)
- 031L0049/Bundesministerium für Bildung und Forschung (BMBF)
- 031L0052/Bundesministerium für Bildung und Forschung (BMBF)
- 031L0256B/Bundesministerium für Bildung und Forschung (BMBF)
- 031L0257D/Bundesministerium für Bildung und Forschung (BMBF)
- 031L0256C/Bundesministerium für Bildung und Forschung (BMBF)
- 031L0258E/Bundesministerium für Bildung und Forschung (BMBF)
- 457840828/Deutsche Forschungsgemeinschaft (DFG)
- Open Access Publication Fund/University of Freiburg
- bwHPC/Baden-Württemberg
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

