Iron accumulation induced by hepcidin1 knockout accelerates the progression of aging osteoporosis
- PMID: 38216929
- PMCID: PMC10785403
- DOI: 10.1186/s13018-024-04535-z
Iron accumulation induced by hepcidin1 knockout accelerates the progression of aging osteoporosis
Abstract
Objective: Iron accumulation is associated with osteoporosis. This study aims to explore the effect of chronic iron accumulation induced by hepcidin1 deficiency on aging osteoporosis.
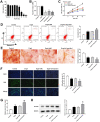
Methods: Iron accumulation in hepcidin1 knockout aging mice was assessed by atomic absorption spectroscopy and Perl's staining. Bone microarchitecture was observed using Micro-CT. Hepcidin, ferritin, oxidative stress, and markers of bone turnover in serum were detected by enzyme-linked immunosorbent assay. Bone formation and resorption markers were measured by real-time quantitative PCR. Cell aging was induced by D-galactose treatment. CCK-8, flow cytometry, EdU assays, and Alizarin red staining were performed to reveal the role of hepcidin1 knockout in cell model. Iron Colorimetric Assay Kit and western blot were applied to detect iron and ferritin levels in cells, respectively.
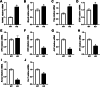
Results: In hepcidin1-knockout mice, the ferritin and iron contents in liver and tibia were significantly increased. Iron accumulation induced by hepcidin1 knockout caused a phenotype of low bone mass and deteriorated bone microarchitecture. Osteogenic marker was decreased and osteoclast marker was increased in mice, accompanied by increased oxidative stress level. The mRNA expression levels of osteoclast differentiation markers (RANKL, Mmp9, OPG, Trap, and CTSK) were up-regulated, while bone formation markers (OCN, ALP, Runx2, SP7, and Col-1) were down-regulated in model group, compared to wild type mice. In vitro, hepcidin1 knockdown inhibited proliferation and osteogenic differentiation, while promoted apoptosis, with increased levels of iron and ferritin.
Conclusion: Iron accumulation induced by hepcidin1 deficiency aggravates the progression of aging osteoporosis via inhibiting osteogenesis and promoting osteoclast genesis.
Keywords: Aged mice; Hepcidin; Iron; Osteoporosis.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interest.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

