Unraveling the dynamic mechanisms of natural killer cells in viral infections: insights and implications
- PMID: 38216935
- PMCID: PMC10785350
- DOI: 10.1186/s12985-024-02287-0
Unraveling the dynamic mechanisms of natural killer cells in viral infections: insights and implications
Abstract
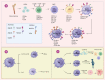
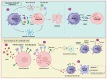
Viruses pose a constant threat to human well-being, necessitating the immune system to develop robust defenses. Natural killer (NK) cells, which play a crucial role in the immune system, have become recognized as vital participants in protecting the body against viral infections. These remarkable innate immune cells possess the unique ability to directly recognize and eliminate infected cells, thereby contributing to the early control and containment of viral pathogens. However, recent research has uncovered an intriguing phenomenon: the alteration of NK cells during viral infections. In addition to their well-established role in antiviral defense, NK cells undergo dynamic changes in their phenotype, function, and regulatory mechanisms upon encountering viral pathogens. These alterations can significantly impact the effectiveness of NK cell responses during viral infections. This review explores the multifaceted role of NK cells in antiviral immunity, highlighting their conventional effector functions as well as the emerging concept of NK cell alteration in the context of viral infections. Understanding the intricate interplay between NK cells and viral infections is crucial for advancing our knowledge of antiviral immune responses and could offer valuable information for the creation of innovative therapeutic approaches to combat viral diseases.
Keywords: Antiviral immunity; Chronic infection; Innate immune response; NK cell alteration; Natural killer cells; Viral infections.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Natural killer cell effector functions in antiviral defense.FEBS J. 2022 Jul;289(14):3982-3999. doi: 10.1111/febs.16073. Epub 2021 Jun 30. FEBS J. 2022. PMID: 34125493 Review.
-
Structural insights into activation of antiviral NK cell responses.Immunol Rev. 2012 Nov;250(1):239-57. doi: 10.1111/j.1600-065X.2012.01168.x. Immunol Rev. 2012. PMID: 23046134 Free PMC article. Review.
-
Viral Evasion of Natural Killer Cell Activation.Viruses. 2016 Apr 12;8(4):95. doi: 10.3390/v8040095. Viruses. 2016. PMID: 27077876 Free PMC article. Review.
-
Natural killer cell responses to viral infection.J Innate Immun. 2011;3(3):274-9. doi: 10.1159/000324176. Epub 2011 Mar 12. J Innate Immun. 2011. PMID: 21411975 Free PMC article. Review.
-
Therapeutic depletion of natural killer cells controls persistent infection.J Virol. 2014 Feb;88(4):1953-60. doi: 10.1128/JVI.03002-13. Epub 2013 Nov 27. J Virol. 2014. PMID: 24284324 Free PMC article.
Cited by
-
CD8+ and CD8- NK Cells and Immune Checkpoint Networks in Peripheral Blood During Healthy Pregnancy.Int J Mol Sci. 2025 Jan 6;26(1):428. doi: 10.3390/ijms26010428. Int J Mol Sci. 2025. PMID: 39796279 Free PMC article.
-
Beyond CAR-T: The rise of CAR-NK cell therapy in asthma immunotherapy.J Transl Med. 2024 Aug 5;22(1):736. doi: 10.1186/s12967-024-05534-8. J Transl Med. 2024. PMID: 39103889 Free PMC article. Review.
-
The Impact of Killer Cell Immunoglobulin-Like Receptors and Human Leukocyte Antigen-E, Human Leukocyte Antigen-G Polymorphisms on Innate Immunity and COVID-19 Severity.J Immunol Res. 2025 May 12;2025:6691437. doi: 10.1155/jimr/6691437. eCollection 2025. J Immunol Res. 2025. PMID: 40391199 Free PMC article.
-
Integrated bulk RNA sequencing and mass cytometry analysis reveal the circulating immune landscape in ischemic and hemorrhagic Moyamoya disease.BMC Immunol. 2025 Mar 10;26(1):19. doi: 10.1186/s12865-025-00699-3. BMC Immunol. 2025. PMID: 40065209 Free PMC article.
-
The immunogenetic basis of severe herpes simplex infections in neonates and children: a review.Pediatr Res. 2025 Mar;97(4):1370-1380. doi: 10.1038/s41390-025-03830-7. Epub 2025 Jan 18. Pediatr Res. 2025. PMID: 39827257 Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

