Long-term inhibition of ODC1 in APP/PS1 mice rescues amyloid pathology and switches astrocytes from a reactive to active state
- PMID: 38216963
- PMCID: PMC10785549
- DOI: 10.1186/s13041-024-01076-8
Long-term inhibition of ODC1 in APP/PS1 mice rescues amyloid pathology and switches astrocytes from a reactive to active state
Abstract
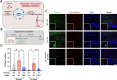
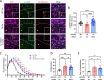
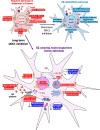
Alzheimer's disease (AD) is characterized by the loss of memory due to aggregation of misphosphorylated tau and amyloid beta (Aβ) plaques in the brain, elevated release of inhibitory neurotransmitter gamma-aminobutyric acid (GABA) and reactive oxygen species from astrocytes, and subsequent neurodegeneration. Recently, it was found that enzyme Ornithine Decarboxylase 1 (ODC1) acts as a bridge between the astrocytic urea cycle and the putrescine-to-GABA conversion pathway in the brain of AD mouse models as well as human patients. In this study, we show that the long-term knockdown of astrocytic Odc1 in APP/PS1 animals was sufficient to completely clear Aβ plaques in the hippocampus while simultaneously switching the astrocytes from a detrimental reactive state to a regenerative active state, characterized by proBDNF expression. Our experiments also reveal an effect of astrocytic ODC1 inhibition on the expression of genes involved in synapse pruning and organization, histone modification, apoptotic signaling and protein processing. These genes are previously known to be associated with astrocytic activation and together create a neuroregeneration-supportive environment in the brain. By inhibiting ODC1 for a long period of 3 months in AD mice, we demonstrate that the beneficial amyloid-clearing process of astrocytes can be completely segregated from the systemically harmful astrocytic response to insult. Our study reports an almost complete clearance of Aβ plaques by controlling an endogenous degradation process, which also modifies the astrocytic state to create a regeneration-supportive environment in the brain. These findings present the potential of modulating astrocytic clearance of Aβ as a powerful therapeutic strategy against AD.
Keywords: Alzheimer’s disease; Ornithine decarboxylase 1; Reactive astrocytes.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Pegg AE. Polyamine metabolism and its importance in neoplastic growth and as a target for chemotherapy. Can Res. 1988;48(4):759–774. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

