Unraveling and Harnessing the Immune Response at the Cell-Biomaterial Interface for Tissue Engineering Purposes
- PMID: 38217464
- PMCID: PMC11468937
- DOI: 10.1002/adhm.202301939
Unraveling and Harnessing the Immune Response at the Cell-Biomaterial Interface for Tissue Engineering Purposes
Abstract
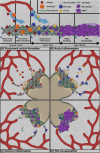
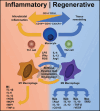
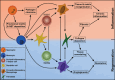
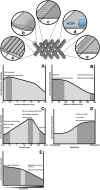
Biomaterials are defined as "engineered materials" and include a range of natural and synthetic products, designed for their introduction into and interaction with living tissues. Biomaterials are considered prominent tools in regenerative medicine that support the restoration of tissue defects and retain physiologic functionality. Although commonly used in the medical field, these constructs are inherently foreign toward the host and induce an immune response at the material-tissue interface, defined as the foreign body response (FBR). A strong connection between the foreign body response and tissue regeneration is suggested, in which an appropriate amount of immune response and macrophage polarization is necessary to trigger autologous tissue formation. Recent developments in this field have led to the characterization of immunomodulatory traits that optimizes bioactivity, the integration of biomaterials and determines the fate of tissue regeneration. This review addresses a variety of aspects that are involved in steering the inflammatory response, including immune cell interactions, physical characteristics, biochemical cues, and metabolomics. Harnessing the advancing knowledge of the FBR allows for the optimization of biomaterial-based implants, aiming to prevent damage of the implant, improve natural regeneration, and provide the tools for an efficient and successful in vivo implantation.
Keywords: biofabrication; biomaterials; foreign body response; immune response.
© 2024 The Authors. Advanced Healthcare Materials published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Vacanti J., Vacanti C., 2000, Ch.1, pp. 3–7.
-
- Vacanti J., Vacanti C. A., 2007, Ch.1, pp. 3–6.
-
- Salgado A. J., Oliveira J. M., Martins A., Teixeira F. G., Silva N. A., Neves N. M., Sousa N., Reis R. L., Int. Rev. Neurobiol. 2013, 108, 1. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

