Eliminating senescent cells by white adipose tissue-targeted senotherapy alleviates age-related hepatic steatosis through decreasing lipolysis
- PMID: 38217637
- PMCID: PMC11009221
- DOI: 10.1007/s11357-024-01068-5
Eliminating senescent cells by white adipose tissue-targeted senotherapy alleviates age-related hepatic steatosis through decreasing lipolysis
Abstract
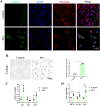
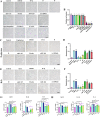
Cellular senescence is an important risk factor in the development of hepatic steatosis. Senolytics present therapeutic effects on age-related hepatic steatosis without eliminating senescent hepatocytes directly. Therefore, it highlights the need to find senolytics' therapeutic targets. Dysfunction of adipose tissue underlies the critical pathogenesis of lipotoxicity in the liver. However, the correlation between adipose tissue and hepatic steatosis during aging and its underlying molecular mechanism remains poorly understood. We explored the correlation between white adipose tissue (WAT) and the liver during aging and evaluated the effect of lipolysis of aged WAT on hepatic steatosis and hepatocyte senescence. We screened out the ideal senolytics for WAT and developed a WAT-targeted delivery system for senotherapy. We assessed senescence and lipolysis of WAT and hepatic lipid accumulation after treatment. The results displayed that aging accelerated cellular senescence and facilitated lipolysis of WAT. Free fatty acids (FFAs) generated by WAT during aging enhanced hepatic steatosis and induced hepatocyte senescence. The combined usage of dasatinib and quercetin was screened out as the ideal senolytics to eliminate senescent cells in WAT. To minimize non-specific distribution and enhance the effectiveness of senolytics, liposomes decorated with WAT affinity peptide P3 were constructed for senotherapy in vivo. In vivo study, WAT-targeted treatment eliminated senescent cells in WAT and reduced lipolysis, resulting in the alleviation of hepatic lipid accumulation and hepatocyte senescence when compared to non-targeted treatment, providing a novel tissue-targeted, effective and safe senotherapy for age-related hepatic steatosis.
Keywords: Cellular senescence; Hepatic steatosis; Lipolysis; Senolytics; White adipose tissue.
© 2024. The Author(s), under exclusive licence to American Aging Association.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

