Effects of Exogenous GIP and GLP-2 on Bone Turnover in Individuals With Type 2 Diabetes
- PMID: 38217866
- PMCID: PMC11180509
- DOI: 10.1210/clinem/dgae022
Effects of Exogenous GIP and GLP-2 on Bone Turnover in Individuals With Type 2 Diabetes
Abstract
Context: Individuals with type 2 diabetes (T2D) have an increased risk of bone fractures despite normal or increased bone mineral density. The underlying causes are not well understood but may include disturbances in the gut-bone axis, in which both glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-2 (GLP-2) are regulators of bone turnover. Thus, in healthy fasting participants, both exogenous GIP and GLP-2 acutely reduce bone resorption.
Objective: The objective of this study was to investigate the acute effects of subcutaneously administered GIP and GLP-2 on bone turnover in individuals with T2D.
Methods: We included 10 men with T2D. Participants met fasting in the morning on 3 separate test days and were injected subcutaneously with GIP, GLP-2, or placebo in a randomized crossover design. Blood samples were drawn at baseline and regularly after injections. Bone turnover was estimated by circulating levels of collagen type 1 C-terminal telopeptide (CTX), procollagen type 1 N-terminal propeptide (P1NP), sclerostin, and PTH.
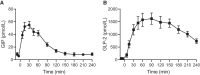
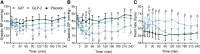
Results: GIP and GLP-2 significantly reduced CTX to (mean ± SEM) 66 ± 7.8% and 74 ± 5.9% of baseline, respectively, compared with after placebo (P = .001). In addition, P1NP and sclerostin increased acutely after GIP whereas a decrease in P1NP was seen after GLP-2. PTH levels decreased to 67 ± 2.5% of baseline after GLP-2 and to only 86 ± 3.4% after GIP.
Conclusion: Subcutaneous GIP and GLP-2 affect CTX and P1NP in individuals with T2D to the same extent as previously demonstrated in healthy individuals.
Keywords: CTX; P1NP; bone turnover; glucagon-like peptide-2 (GLP-2); glucose-dependent insulinotropic polypeptide (GIP); gut-bone axis.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Endocrine Society.
Figures


References
-
- Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes-a meta-analysis. Osteoporos Int. 2007;18(4):427‐444. - PubMed
-
- Schwartz AV, Sellmeyer DE, Ensrud KE, et al. Older women with diabetes have an increased risk of fracture: a prospective study. J Clin Endocrinol Metab. 2001;86(1):32‐38. - PubMed
-
- Janghorbani M, Van Dam RM, Willett WC, Hu FB. Systematic review of type 1 and type 2 diabetes mellitus and risk of fracture. Am J Epidemiol. 2007;166(5):495‐505. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

