Primary, seminal and lateral roots of maize show type-specific growth and hydraulic responses to water deficit
- PMID: 38217868
- PMCID: PMC10980523
- DOI: 10.1093/plphys/kiad675
Primary, seminal and lateral roots of maize show type-specific growth and hydraulic responses to water deficit
Abstract
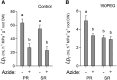
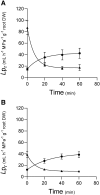
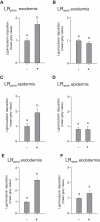
The water uptake capacity of a root system is determined by its architecture and hydraulic properties, which together shape the root hydraulic architecture. Here, we investigated root responses to water deficit (WD) in seedlings of a maize (Zea mays) hybrid line (B73H) grown in hydroponic conditions, taking into account the primary root (PR), the seminal roots (SR), and their respective lateral roots. WD was induced by various polyethylene glycol concentrations and resulted in dose-dependent inhibitions of axial and lateral root growth, lateral root formation, and hydraulic conductivity (Lpr), with slightly distinct sensitivities to WD between PR and SR. Inhibition of Lpr by WD showed a half-time of 5 to 6 min and was fully (SR) or partially (PR) reversible within 40 min. In the two root types, WD resulted in reduced aquaporin expression and activity, as monitored by mRNA abundance of 13 plasma membrane intrinsic protein (ZmPIP) isoforms and inhibition of Lpr by sodium azide, respectively. An enhanced suberization/lignification of the epi- and exodermis was observed under WD in axial roots and in lateral roots of the PR but not in those of SR. Inverse modeling revealed a steep increase in axial conductance in root tips of PR and SR grown under WD that may be due to the decreased growth rate of axial roots in these conditions. Overall, our work reveals that these root types show quantitative differences in their anatomical, architectural, and hydraulic responses to WD, in terms of sensitivity, amplitude and reversibility. This distinct functionalization may contribute to integrative acclimation responses of whole root systems to soil WD.
© The Author(s) 2024. Published by Oxford University Press on behalf of American Society of Plant Biologists.
Conflict of interest statement
Conflict of interest statement. None declared.
Figures




Similar articles
-
Natural variation of maize root hydraulic architecture underlies highly diverse water uptake capacities.Plant Physiol. 2023 Jul 3;192(3):2404-2418. doi: 10.1093/plphys/kiad213. Plant Physiol. 2023. PMID: 37052178 Free PMC article.
-
Hydraulic conductivity of soil-grown lupine and maize unbranched roots and maize root-shoot junctions.J Plant Physiol. 2018 Aug;227:31-44. doi: 10.1016/j.jplph.2017.12.019. Epub 2018 Jan 31. J Plant Physiol. 2018. PMID: 29395124
-
Abscisic Acid Coordinates Dose-Dependent Developmental and Hydraulic Responses of Roots to Water Deficit.Plant Physiol. 2019 Aug;180(4):2198-2211. doi: 10.1104/pp.18.01546. Epub 2019 Jun 4. Plant Physiol. 2019. PMID: 31164395 Free PMC article.
-
Steep, cheap and deep: an ideotype to optimize water and N acquisition by maize root systems.Ann Bot. 2013 Jul;112(2):347-57. doi: 10.1093/aob/mcs293. Epub 2013 Jan 17. Ann Bot. 2013. PMID: 23328767 Free PMC article. Review.
-
Quantification of root water uptake and redistribution using neutron imaging: a review and future directions.Plant J. 2022 Jul;111(2):348-359. doi: 10.1111/tpj.15839. Epub 2022 Jun 15. Plant J. 2022. PMID: 35603461 Review.
Cited by
-
Natural variation in a cortex/epidermis-specific transcription factor bZIP89 determines lateral root development and drought resilience in maize.Sci Adv. 2025 Apr 11;11(15):eadt1113. doi: 10.1126/sciadv.adt1113. Epub 2025 Apr 11. Sci Adv. 2025. PMID: 40215297 Free PMC article.
-
The transcription factors DOF4.6 and XND1 jointly regulate root hydraulics and drought responses in Arabidopsis.Plant Cell. 2025 Apr 2;37(4):koaf083. doi: 10.1093/plcell/koaf083. Plant Cell. 2025. PMID: 40262770
-
Root system ideotypes: what is the potential for breeding drought-tolerant grapevine rootstocks?J Exp Bot. 2025 Aug 5;76(11):2970-2984. doi: 10.1093/jxb/eraf006. J Exp Bot. 2025. PMID: 39787137 Free PMC article. Review.
-
A maize landrace introgression library reveals a negative effect of root-to-shoot ratio on water-use efficiency.Plant Genome. 2025 Jun;18(2):e70036. doi: 10.1002/tpg2.70036. Plant Genome. 2025. PMID: 40281664 Free PMC article.
References
-
- Ahmed MA, Zarebanadkouki M, Kaestner A, Carminati A. Measurements of water uptake of maize roots: the key function of lateral roots. Plant Soil. 2016:398(1–2):59–77. 10.1007/s11104-015-2639-6 - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

