Novel loss-of-function variants expand ABCC9-related intellectual disability and myopathy syndrome
- PMID: 38217872
- PMCID: PMC11068106
- DOI: 10.1093/brain/awae010
Novel loss-of-function variants expand ABCC9-related intellectual disability and myopathy syndrome
Abstract
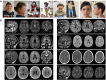
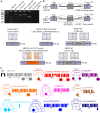
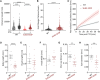
Loss-of-function mutation of ABCC9, the gene encoding the SUR2 subunit of ATP sensitive-potassium (KATP) channels, was recently associated with autosomal recessive ABCC9-related intellectual disability and myopathy syndrome (AIMS). Here we identify nine additional subjects, from seven unrelated families, harbouring different homozygous loss-of-function variants in ABCC9 and presenting with a conserved range of clinical features. All variants are predicted to result in severe truncations or in-frame deletions within SUR2, leading to the generation of non-functional SUR2-dependent KATP channels. Affected individuals show psychomotor delay and intellectual disability of variable severity, microcephaly, corpus callosum and white matter abnormalities, seizures, spasticity, short stature, muscle fatigability and weakness. Heterozygous parents do not show any conserved clinical pathology but report multiple incidences of intra-uterine fetal death, which were also observed in an eighth family included in this study. In vivo studies of abcc9 loss-of-function in zebrafish revealed an exacerbated motor response to pentylenetetrazole, a pro-convulsive drug, consistent with impaired neurodevelopment associated with an increased seizure susceptibility. Our findings define an ABCC9 loss-of-function-related phenotype, expanding the genotypic and phenotypic spectrum of AIMS and reveal novel human pathologies arising from KATP channel dysfunction.
Keywords: ABCC9; KATP channels; SUR2; neurodevelopmental disorder.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
The authors report no competing interests.
Figures

References
-
- Nichols CG. KATP channels as molecular sensors of cellular metabolism. Nature. 2006;440:470–476. - PubMed
-
- Zerangue N, Schwappach B, Jan YN, Jan LY. A new ER trafficking signal regulates the subunit stoichiometry of plasma membrane K(ATP) channels. Neuron. 1999;22:537–548. - PubMed
-
- Schwappach B, Zerangue N, Jan YN, Jan LY. Molecular basis for K(ATP) assembly: Transmembrane interactions mediate association of a K+ channel with an ABC transporter. Neuron. 2000;26:155–167. - PubMed
-
- Inagaki N, Gonoi T, Clement JP IV, et al. Reconstitution of IKATP: An inward rectifier subunit plus the sulfonylurea receptor. Science. 1995;270:1166–1170. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

