Efficacy, safety and genomic analysis of SCT200, an anti-EGFR monoclonal antibody, in patients with fluorouracil, irinotecan and oxaliplatin refractory RAS and BRAF wild-type metastatic colorectal cancer: a phase Ⅱ study
- PMID: 38217945
- PMCID: PMC10826138
- DOI: 10.1016/j.ebiom.2024.104966
Efficacy, safety and genomic analysis of SCT200, an anti-EGFR monoclonal antibody, in patients with fluorouracil, irinotecan and oxaliplatin refractory RAS and BRAF wild-type metastatic colorectal cancer: a phase Ⅱ study
Abstract
Background: Limited therapeutic options are available for metastatic colorectal cancer (mCRC) patients after failure of first- and second-line therapies, representing an unmet medical need for novel therapies.
Methods: This is an open-label, single arm, multicenter, phase Ⅱ study aiming to perform the efficacy, safety and genomic analysis of SCT200, a noval fully humanized IgG1 anti-epidermal growth factor receptor (EGFR) monoclonal antibody, in patients with fluorouracil, irinotecan and oxaliplatin refractory RAS and BRAF wild-type mCRC. SCT200 (6 mg/kg) was given weekly for the first six weeks, followed by a higher dose of 8 mg/kg every two weeks until disease progression or unacceptable toxicity. Primary endpoint was independent review committee (IRC)-assessed objective response rate (ORR) and secondary endpoints included ORR in patients with left-sided tumor, disease control rate (DCR), duration of response (DoR), time to response (TTR), progression-free survival (PFS), overall survival (OS) and safety.
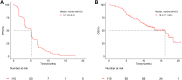
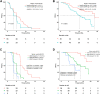
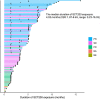
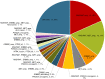
Findings: From February 12, 2018 to December 1, 2019, a total of 110 patients aged between 26 and 77 years (median: 55; interquartile range [IQR]: 47-63) with fluorouracil, oxaliplatin, and irinotecan refractory RAS and BRAF wild-type mCRC were enrolled from 22 hospitals in China. As the data cut-off date on May 15, 2020, the IRC-assessed ORR and DCR was 31% (34/110, 95% confidence interval [CI] 22-40%) and 75% (82/110, 95% CI 65-82%), respectively. Thirty one percent (34/110) patients achieved confirmed partial response (PR). The median PFS and median OS were 5.1 months (95% CI 3.4-5.2) and 16.2 months (95% CI 11.1-not available [NA]), respectively. The most common ≥ grade 3 treatment-related adverse events (TRAEs) were hypomagnesemia (17%, 19/110) and acneiform dermatitis (11%, 12/110). No deaths occurred. Genomic analysis suggested positive association between MYC amplification and patients' response (P = 0.0058). RAS/RAF mutation and MET amplification were the most frequently detected resistance mechanisms. Patients with high circulating tumor DNA (ctDNA) at baseline or without ctDNA clearance at the 7th week after the first dose of SCT200 administration before receiving SCT200 had worse PFS and OS.
Interpretation: SCT200 exhibited promising clinical efficacy and manageable safety profiles in RAS and BRAF wild-type mCRC patients progressed on fluorouracil, irinotecan and oxaliplatin treatment. The baseline ctDNA and ctDNA clearance status at the 7th week after the first dose of SCT200 administration before receiving SCT200 could be a potential prognostic biomarker for RAS and BRAF wild-type mCRC patients with SCT200 therapy.
Funding: This study was sponsored by Sinocelltech Ltd., Beijing, China and partly supported by the National Science and Technology Major Project for Key New Drug Development (2019ZX09732001-006, 2017ZX09304015).
Keywords: Anti-epidermal growth factor receptor; BRAF; Circulating tumor DNA; Metastatic colorectal cancer; Monoclonal antibody; RAS.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests Liangzhi Xie, Wenlin Gai, and Yan Wang are employees of Sinocelltech Ltd., Beijing, China. Peng Meng is employee of Burning Rock Biotech, Shanghai, China. The other authors declare that there is no conflict of interests regarding the publication of this article.
Figures






References
-
- Food and Drug Administration 2004. https://wwwaccessdatafdagov/drugsatfda_docs/appletter/2004/125084ltrpdf
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

