The oxidized phospholipid PGPC impairs endothelial function by promoting endothelial cell ferroptosis via FABP3
- PMID: 38218337
- PMCID: PMC10864338
- DOI: 10.1016/j.jlr.2024.100499
The oxidized phospholipid PGPC impairs endothelial function by promoting endothelial cell ferroptosis via FABP3
Abstract
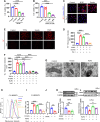
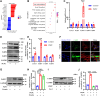
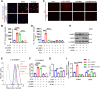
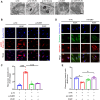
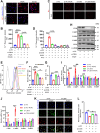
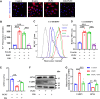
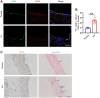
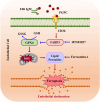
Ferroptosis is a novel cell death mechanism that is mediated by iron-dependent lipid peroxidation. It may be involved in atherosclerosis development. Products of phospholipid oxidation play a key role in atherosclerosis. 1-palmitoyl-2-glutaroyl-sn-glycero-3-phosphocholine (PGPC) is a phospholipid oxidation product present in atherosclerotic lesions. It remains unclear whether PGPC causes atherosclerosis by inducing endothelial cell ferroptosis. In this study, human umbilical vein endothelial cells (HUVECs) were treated with PGPC. Intracellular levels of ferrous iron, lipid peroxidation, superoxide anions (O2•-), and glutathione were detected, and expression of fatty acid binding protein-3 (FABP3), glutathione peroxidase 4 (GPX4), and CD36 were measured. Additionally, the mitochondrial membrane potential (MMP) was determined. Aortas from C57BL6 mice were isolated for vasodilation testing. Results showed that PGPC increased ferrous iron levels, the production of lipid peroxidation and O2•-, and FABP3 expression. However, PGPC inhibited the expression of GPX4 and glutathione production and destroyed normal MMP. These effects were also blocked by ferrostatin-1, an inhibitor of ferroptosis. FABP3 silencing significantly reversed the effect of PGPC. Furthermore, PGPC stimulated CD36 expression. Conversely, CD36 silencing reversed the effects of PGPC, including PGPC-induced FABP3 expression. Importantly, E06, a direct inhibitor of the oxidized 1-palmitoyl-2-arachidonoyl-phosphatidylcholine IgM natural antibody, inhibited the effects of PGPC. Finally, PGPC impaired endothelium-dependent vasodilation, ferrostatin-1 or FABP3 inhibitors inhibited this impairment. Our data demonstrate that PGPC impairs endothelial function by inducing endothelial cell ferroptosis through the CD36 receptor to increase FABP3 expression. Our findings provide new insights into the mechanisms of atherosclerosis and a therapeutic target for atherosclerosis.
Keywords: Atherosclerosis; CD36; Endothelial function; Fatty acid binding protein-3; Oxidized lipids; PGPC.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures

References
-
- Ou Z.J., Chen J., Dai W.P., Liu X., Yang Y.K., Li Y., et al. 25-Hydroxycholesterol impairs endothelial function and vasodilation by uncoupling and inhibiting endothelial nitric oxide synthase. Am. J. Physiol. Endocrinol. Metab. 2016;311:E781–E790. - PubMed
-
- Yan F.X., Li H.M., Li S.X., He S.H., Dai W.P., Li Y., et al. The oxidized phospholipid POVPC impairs endothelial function and vasodilation via uncoupling endothelial nitric oxide synthase. J. Mol. Cell. Cardiol. 2017;112:40–48. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

