Antibody blockade of activin type II receptors preserves skeletal muscle mass and enhances fat loss during GLP-1 receptor agonism
- PMID: 38218536
- PMCID: PMC10832506
- DOI: 10.1016/j.molmet.2024.101880
Antibody blockade of activin type II receptors preserves skeletal muscle mass and enhances fat loss during GLP-1 receptor agonism
Abstract
Objective: Glucagon-like peptide 1 (GLP-1) receptor agonists reduce food intake, producing remarkable weight loss in overweight and obese individuals. While much of this weight loss is fat mass, there is also a loss of lean mass, similar to other approaches that induce calorie deficit. Targeting signaling pathways that regulate skeletal muscle hypertrophy is a promising avenue to preserve lean mass and modulate body composition. Myostatin and Activin A are TGFβ-like ligands that signal via the activin type II receptors (ActRII) to antagonize muscle growth. Pre-clinical and clinical studies demonstrate that ActRII blockade induces skeletal muscle hypertrophy and reduces fat mass. In this manuscript, we test the hypothesis that combined ActRII blockade and GLP-1 receptor agonism will preserve muscle mass, leading to improvements in skeletomuscular and metabolic function and enhanced fat loss.
Methods: In this study, we explore the therapeutic potential of bimagrumab, a monoclonal antibody against ActRII, to modify body composition alone and during weight loss induced by GLP-1 receptor agonist semaglutide in diet-induced obese mice. Mechanistically, we define the specific role of the anabolic kinase Akt in mediating the hypertrophic muscle effects of ActRII inhibition in vivo.
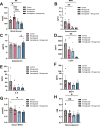
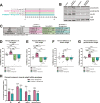
Results: Treatment of obese mice with bimagrumab induced a ∼10 % increase in lean mass while simultaneously decreasing fat mass. Daily treatment of obese mice with semaglutide potently decreased body weight; this included a significant decrease in both muscle and fat mass. Combination treatment with bimagrumab and semaglutide led to superior fat mass loss while simultaneously preserving lean mass despite reduced food intake. Treatment with both drugs was associated with improved metabolic outcomes, and increased lean mass was associated with improved exercise performance. Deletion of both Akt isoforms in skeletal muscle modestly reduced, but did not prevent, muscle hypertrophy driven by ActRII inhibition.
Conclusions: Collectively, these data demonstrate that blockade of ActRII signaling improves body composition and metabolic parameters during calorie deficit driven by GLP-1 receptor agonism and demonstrate the existence of Akt-independent pathways supporting muscle hypertrophy in the absence of ActRII signaling.
Keywords: Activin type II receptor (ActRII); Akt; Body composition; Glucagon-like peptide 1 (GLP-1); Obesity; Weight loss.
Copyright © 2024 The Author(s). Published by Elsevier GmbH.. All rights reserved.
Conflict of interest statement
Declaration of competing interest None declared.
Figures




References
-
- N.d. World Obesity Federation . 2023. World obesity atlas.https://data.worldobesity.org/publications/?cat=19

