Extracellular derivatives for bone metabolism
- PMID: 38218580
- PMCID: PMC11674789
- DOI: 10.1016/j.jare.2024.01.011
Extracellular derivatives for bone metabolism
Abstract
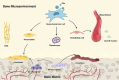
Background: Bone metabolism can maintain the normal homeostasis and function of bone tissue. Once the bone metabolism balance is broken, it will cause osteoporosis, osteoarthritis, bone defects, bone tumors, or other bone diseases. However, such orthopedic diseases still have many limitations in clinical treatment, such as drug restrictions, drug tolerance, drug side effects, and implant rejection.
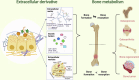
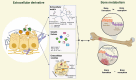
Aim of review: In complex bone therapy and bone regeneration, extracellular derivatives have become a promising research focus to solve the problems of bone metabolic diseases. These derivatives, which include components such as extracellular matrix, growth factors, and extracellular vesicles, have significant therapeutic potential. It has the advantages of good biocompatibility, low immune response, and dynamic demand for bone tissue. The purpose of this review is to provide a comprehensive perspective on extracellular derivatives for bone metabolism and elucidate the intrinsic properties and versatility of extracellular derivatives. Further discussion of them as innovative advanced orthopedic materials for improving the effectiveness of bone therapy and regeneration processes.
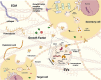
Key scientific concepts of review: In this review, we first listed the types and functions of three extracellular derivatives. Then, we discussed the effects of extracellular derivatives of different cell sources on bone metabolism. Subsequently, we collected applications of extracellular derivatives in the treatment of bone metabolic diseases and summarized the advantages and challenges of extracellular derivatives in clinical applications. Finally, we prospected the extracellular derivatives in novel orthopedic materials and clinical applications. We hope that the comprehensive understanding of extracellular derivatives in bone metabolism will provide new solutions to bone diseases.
Keywords: Bone regeneration; Clinical application; Extracellular matrix; Extracellular vesicles; Osteoporosis.
Copyright © 2023. Published by Elsevier B.V.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

