Brain-targeted delivery of neuroprotective survival gene minimizing hematopoietic cell contamination: implications for Parkinson's disease treatment
- PMID: 38218903
- PMCID: PMC10790275
- DOI: 10.1186/s12967-023-04816-x
Brain-targeted delivery of neuroprotective survival gene minimizing hematopoietic cell contamination: implications for Parkinson's disease treatment
Abstract
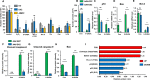
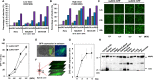
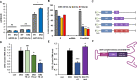
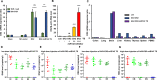
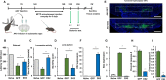
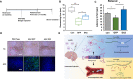
Background: Neurodegenerative diseases, including Parkinson's disease, Amyotropic Lateral Sclerosis (ALS) and Alzheimer's disease, present significant challenges for therapeutic development due to drug delivery restrictions and toxicity concerns. Prevailing strategies often employ adeno-associated viral (AAV) vectors to deliver neuroprotective survival genes directly into the central nervous system (CNS). However, these methods have been limited by triggering immunogenic responses and risk of tumorigenicity, resulting from overexpression of survival genes in peripheral blood mononuclear cells (PBMC), thereby increasing the risk of tumorigenicity in specific immune cells. Thus, by coding selectively suppressive microRNA (miRNA) target sequences in AAV genome, we designed CNS-targeted neuroprotective gene expression vector system without leakage to blood cells.
Methods: To minimize the potential for transgene contamination in the blood, we designed a CNS-specific AAV system. Our system utilized a self-complementary AAV (scAAV), encoding a quadruple repeated target sequence of the hematopoietic cell-specific miR142-3p at the 3' untranslated region (UTR). As a representative therapeutic survival gene for Parkinson's disease treatment, we integrated DX2, an antagonistic splice variant of the apoptotic gene AIMP2, known to be implicated in Parkinson's disease, into the vector.
Results: This configuration ensured that transgene expression was stringently localized to the CNS, even if the vector found its way into the blood cells. A single injection of scAAV-DX2 demonstrated marked improvement in behavior and motor activity in animal models of Parkinson's disease induced by either Rotenone or 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Importantly, comprehensive preclinical data adhering to Good Laboratory Practice (GLP) standards revealed no adverse effects in the treated animals.
Conclusions: Our CNS-specific vector system, which encodes a survival transgene DX2, signifies a promising avenue for safe gene therapy, avoiding unintended expression of survival gene in blood cells, applicable to various neurodegenerative diseases.
Keywords: AIMP2 splicing variants; Adeno-associated virus; DX2; Gene therapy; Parkinson’s disease; miRNA.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Pre-existing immunity to adeno-associated virus (AAV)2 limits transgene expression following intracerebral AAV2-based gene delivery in a 6-hydroxydopamine model of Parkinson's disease.J Gene Med. 2014 Sep-Oct;16(9-10):300-8. doi: 10.1002/jgm.2779. J Gene Med. 2014. PMID: 25303717
-
Self-complementary adeno-associated virus serotype 2 vector: global distribution and broad dispersion of AAV-mediated transgene expression in mouse brain.Mol Ther. 2003 Dec;8(6):911-7. doi: 10.1016/j.ymthe.2003.08.021. Mol Ther. 2003. PMID: 14664793
-
AAV-aMTD-Parkin, a therapeutic gene delivery cargo, enhances motor and cognitive functions in Parkinson's and Alzheimer's diseases.Pharmacol Res. 2024 Oct;208:107326. doi: 10.1016/j.phrs.2024.107326. Epub 2024 Jul 26. Pharmacol Res. 2024. PMID: 39069196
-
Towards a neuroprotective gene therapy for Parkinson's disease: use of adenovirus, AAV and lentivirus vectors for gene transfer of GDNF to the nigrostriatal system in the rat Parkinson model.Brain Res. 2000 Dec 15;886(1-2):82-98. doi: 10.1016/s0006-8993(00)02915-2. Brain Res. 2000. PMID: 11119690 Review.
-
Recombinant AAV-mediated gene delivery to the central nervous system.J Gene Med. 2004 Feb;6 Suppl 1:S212-22. doi: 10.1002/jgm.506. J Gene Med. 2004. PMID: 14978764 Review.
Cited by
-
The Dual Role of Survival Genes in Neurons and Cancer Cells: a Strategic Clinical Application of DX2 in Neurodegenerative Diseases and Cancer.Biomol Ther (Seoul). 2025 Jan 1;33(1):75-85. doi: 10.4062/biomolther.2024.138. Epub 2024 Dec 23. Biomol Ther (Seoul). 2025. PMID: 39711064 Free PMC article. Review.
References
-
- Kaplitt MG, Feigin A, Tang C, Fitzsimons HL, Mattis P, Lawlor PA, Bland RJ, Young D, Strybing K, Eidelberg D, et al. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson’s disease: an open label, phase I trial. Lancet. 2007;369(9579):2097–2105. doi: 10.1016/S0140-6736(07)60982-9. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

