New strategies for sterilization and preservation of fresh fish skin grafts
- PMID: 38218988
- PMCID: PMC10787751
- DOI: 10.1038/s41598-024-51608-4
New strategies for sterilization and preservation of fresh fish skin grafts
Abstract
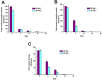
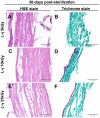
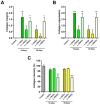
The introduction of fish skin as a biological dressing for treating burns and wounds holds great promise, offering an alternative to existing management strategies. However, the risk of disease transmission is a significant concern. Therefore, this study aimed to examine how established sterilization and preservation procedures affected fish skin grafts' microbiological and histological properties for long-term usage. Lyophilization of the fish skin graft followed by rehydration in normal saline for 15 min did not change the collagen content. Furthermore, gamma irradiation of the lyophilized fish skin graft at different lengths 5, 10, and 25 KGy showed a significant reduction in microbial growth (aerobic bacteria, aerobic yeasts, and fungi) at 15- and 30 days after the irradiation. However, exposure to 10 KGy was found to be the most effective intensity among the different gamma irradiation lengths since it preserved the collagen fiber content and intensity in the lyophilized fish skin grafts at 15- and 30 days after the irradiation. These findings provide efficient preservation and sterilization methods for long-term usage of the fresh Tilapia skin grafts used for biological dressings.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
500-Gray γ-Irradiation May Increase Adhesion Strength of Lyophilized Cadaveric Split-Thickness Skin Graft to Wound Bed.Ann Plast Surg. 2017 Mar;78(3 Suppl 2):S135-S138. doi: 10.1097/SAP.0000000000001019. Ann Plast Surg. 2017. PMID: 28166140
-
Validation of Three Different Sterilization Methods of Tilapia Skin Dressing: Impact on Microbiological Enumeration and Collagen Content.Front Vet Sci. 2020 Dec 23;7:597751. doi: 10.3389/fvets.2020.597751. eCollection 2020. Front Vet Sci. 2020. PMID: 33426019 Free PMC article.
-
Study of tensiometric properties, microbiological and collagen content in nile tilapia skin submitted to different sterilization methods.Cell Tissue Bank. 2018 Sep;19(3):373-382. doi: 10.1007/s10561-017-9681-y. Epub 2018 Jan 29. Cell Tissue Bank. 2018. PMID: 29380095
-
Fish Skin Grafts Versus Alternative Wound Dressings in Wound Care: A Systematic Review of the Literature.Cureus. 2023 Mar 19;15(3):e36348. doi: 10.7759/cureus.36348. eCollection 2023 Mar. Cureus. 2023. PMID: 37082504 Free PMC article. Review.
-
Irradiation as a safety procedure in tissue banking.Cell Tissue Bank. 2005;6(3):201-19. doi: 10.1007/s10561-005-0338-x. Cell Tissue Bank. 2005. PMID: 16151960 Review.
Cited by
-
Collagen-Based Products in Wound, Skin, and Health Care.Adv Wound Care (New Rochelle). 2025 Jul 28:10.1177/21621918251361118. doi: 10.1177/21621918251361118. Online ahead of print. Adv Wound Care (New Rochelle). 2025. PMID: 40720447 Free PMC article. Review.
-
Effects of cold storage on double integrating sphere optical property measurements of porcine dermis and subcutaneous fat from 400 to 1100 nm.J Biomed Opt. 2025 Jan;30(1):015001. doi: 10.1117/1.JBO.30.1.015001. Epub 2025 Jan 22. J Biomed Opt. 2025. PMID: 39845727 Free PMC article.
-
Comparative evaluation of fresh and lyophilized Nile tilapia fish skin for enhancing wound healing in a donkey model.Vet Res Commun. 2025 Jul 23;49(5):262. doi: 10.1007/s11259-025-10821-w. Vet Res Commun. 2025. PMID: 40699393 Free PMC article.
References
-
- Costa BA, Lima Júnior EM, de Moraes Filho MO, Fechine FV, de Moraes MEA, Silva Júnior FR, do Nascimento Soares MFA, Rocha MBS. Use of Tilapia skin as a Xenograft for pediatric burn treatment: A case report. J. Burn Care Res. Off. Publ. Am. Burn Assoc. 2019;40(5):714–717. doi: 10.1093/jbcr/irz085. - DOI - PubMed
-
- Júnior EML, de Moraes Filho MO, Costa BA, Alves APNN, de Moraes MEA, do Nascimento Uchôa AM, Martins CB, de Jesus Pinheiro Gomes Bandeira T, Rodrigues FAR, Paier CRK. Lyophilised tilapia skin as a xenograft for superficial partial thickness burns: A novel preparation and storage technique. J. Wound Care. 2020;29(10):598–602. doi: 10.12968/jowc.2020.29.10.598. - DOI - PubMed
-
- Lima Júnior EM, de Moraes Filho MO, Costa BA, Fechine FV, Rocha MBS, Vale ML, Diógenes AKL, Uchôa AMN, Silva Júnior FR, Martins CB. A randomized comparison study of lyophilized Nile tilapia skin and silver-impregnated sodium carboxymethylcellulose for the treatment of superficial partial-thickness burns. J. Burn Care Res. 2021;42(1):41–48. doi: 10.1093/jbcr/iraa099. - DOI - PubMed
-
- Lima Junior EM, de Moraes Filho MO, Costa BA, Fechine FV, Vale ML, Diogenes AKL, Neves KRT, Uchoa AMN, Soares MFAN, de Moraes MEA. Nile tilapia fish skin-based wound dressing improves pain and treatment-related costs of superficial partial-thickness burns: A phase III randomized controlled trial. Plast. Reconstr. Surg. 2021;147(5):1189–1198. doi: 10.1097/PRS.0000000000007895. - DOI - PubMed
-
- Lima Júnior EM, De Moraes Filho MO, Costa BA, Rohleder AVP, Sales Rocha MB, Fechine FV, Forte AJ, Alves APNN, Silva Júnior FR, Martins CB. Innovative burn treatment using tilapia skin as a xenograft: A phase II randomized controlled trial. J. Burn Care Res. 2020;41(3):585–592. doi: 10.1093/jbcr/irz205. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

