Inhibition of xanthine oxidase by allopurinol suppresses HMGB1 secretion and ameliorates experimental asthma
- PMID: 38219573
- PMCID: PMC10825647
- DOI: 10.1016/j.redox.2023.103021
Inhibition of xanthine oxidase by allopurinol suppresses HMGB1 secretion and ameliorates experimental asthma
Abstract
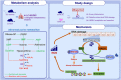
Background: Extracellular high mobility group box 1 (HMGB1) is a key mediator in driving allergic airway inflammation and contributes to asthma. Yet, mechanism of HMGB1 secretion in asthma is poorly defined. Pulmonary metabolic dysfunction is recently recognized as a driver of respiratory pathology. However, the altered metabolic signatures and the roles of metabolic to allergic airway inflammation remain unclear.
Methods: Male C57BL/6 J mice were sensitized and challenged with toluene diisocyanate (TDI) to generate a chemically induced asthma model. Pulmonary untargeted metabolomics was employed. According to results, mice were orally administered allopurinol, a xanthine oxidase (XO) inhibitor. Human bronchial epithelial cells (16HBE) were stimulated by TDI-human serum albumin (HSA).
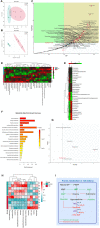
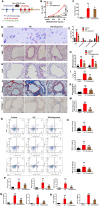
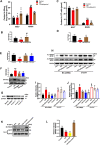
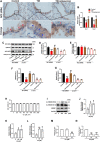
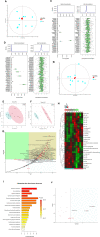
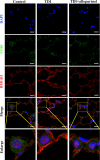
Results: We identified the purine metabolism was the most enriched pathway in TDI-exposed lungs, corresponding to the increase of xanthine and uric acid, products of purine degradation mediated by XO. Inhibition of XO by allopurinol ameliorates TDI-induced oxidative stress and DNA damage, mixed granulocytic airway inflammation and Th1, Th2 and Th17 immunology as well as HMGB1 acetylation and secretion. Mechanistically, HMGB1 acetylation was caused by decreased activation of the NAD+-sirtuin 1 (SIRT1) axis triggered by hyperactivation of the DNA damage sensor poly (ADP-ribose)-polymerase 1 (PARP-1). This was rescued by allopurinol, PARP-1 inhibitor or supplementation with NAD+ precursor in a SIRT1-dependent manner. Meanwhile, allopurinol attenuated Nrf2 defect due to SIRT1 inactivation to help ROS scavenge.
Conclusions: We demonstrated a novel regulation of HMGB1 acetylation and secretion by purine metabolism that is critical for asthma onset. Allopurinol may have therapeutic potential in patients with asthma.
Keywords: Allopurinol; Asthma; HMGB1; Purine metabolism; SIRT1.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare no conflict of interest in relation to this work.
Figures


References
-
- Israel E., Reddel H.K. Severe and difficult-to-treat asthma in adults. N. Engl. J. Med. 2017;377(10):965–976. - PubMed
-
- Agache I., Akdis C.A., Akdis M., et al. EAACI biologicals guidelines—recommendations for severe asthma. Allergy. 2021;76(1):14–44. - PubMed
-
- Chen R., Zhang Q., Chen S., et al. IL-17F, rather than IL-17A, underlies airway inflammation in a steroid-insensitive toluene diisocyanate-induced asthma model. Eur. Respir. J. 2019;53(4) - PubMed
-
- Wang Y., Le Y., Zhao W., et al. Short thymic stromal lymphopoietin attenuates toluene diisocyanate-induced airway inflammation and inhibits high mobility group box 1-receptor for advanced glycation end products and long thymic stromal lymphopoietin expression. Toxicol. Sci. 2017;157(2):276–290. - PubMed
-
- Yao L., Zhao H., Tang H., et al. Blockade of β-catenin signaling attenuates toluene diisocyanate-induced experimental asthma. Allergy. 2017;72(4):579–589. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

