SCMR expert consensus statement for cardiovascular magnetic resonance of patients with a cardiac implantable electronic device
- PMID: 38219955
- PMCID: PMC11211236
- DOI: 10.1016/j.jocmr.2024.100995
SCMR expert consensus statement for cardiovascular magnetic resonance of patients with a cardiac implantable electronic device
Abstract
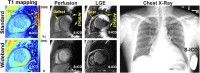
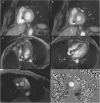
Cardiovascular magnetic resonance (CMR) is a proven imaging modality for informing diagnosis and prognosis, guiding therapeutic decisions, and risk stratifying surgical intervention. Patients with a cardiac implantable electronic device (CIED) would be expected to derive particular benefit from CMR given high prevalence of cardiomyopathy and arrhythmia. While several guidelines have been published over the last 16 years, it is important to recognize that both the CIED and CMR technologies, as well as our knowledge in MR safety, have evolved rapidly during that period. Given increasing utilization of CIED over the past decades, there is an unmet need to establish a consensus statement that integrates latest evidence concerning MR safety and CIED and CMR technologies. While experienced centers currently perform CMR in CIED patients, broad availability of CMR in this population is lacking, partially due to limited availability of resources for programming devices and appropriate monitoring, but also related to knowledge gaps regarding the risk-benefit ratio of CMR in this growing population. To address the knowledge gaps, this SCMR Expert Consensus Statement integrates consensus guidelines, primary data, and opinions from experts across disparate fields towards the shared goal of informing evidenced-based decision-making regarding the risk-benefit ratio of CMR for patients with CIEDs.
Keywords: Cardiac implantable electronic device; Cardiovascular magnetic resonance; Guidelines; MR safety.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Puntmann V.O., Valbuena S., Hinojar R., Petersen S.E., Greenwood J.P., Kramer C.M., Kwong R.Y., McCann G.P., Berry C., Nagel E., et al. Society for Cardiovascular Magnetic Resonance (SCMR) expert consensus for CMR imaging endpoints in clinical research: part I - analytical validation and clinical qualification. J Cardiovasc Magn Reson. 2018;20(1) - PMC - PubMed
-
- Patel H.N., Wang S., Rao S., Singh A., Landeras L., Besser S.A., Carter S., Mishra S., Nishimura T., Shatz D.Y., et al. Impact of wideband cardiac magnetic resonance on diagnosis, decision-making and outcomes in patients with implantable cardioverter defibrillators. Eur Heart J Cardiovasc Imaging. 2022 - PMC - PubMed
-
- Bhuva A.N., Kellman P., Graham A., Ramlall M., Boubertakh R., Feuchter P., Hawkins A., Lowe M., Lambiase P.D., Sekhri N., et al. Clinical impact of cardiovascular magnetic resonance with optimized myocardial scar detection in patients with cardiac implantable devices. Int J Cardiol. 2019;279:72–78. - PubMed
-
- Patel N.J., Edla S., Deshmukh A., Nalluri N., Patel N., Agnihotri K., Patel A., Savani C., Patel N., Bhimani R., et al. Gender, Racial, and Health Insurance Differences in the Trend of Implantable Cardioverter-Defibrillator (ICD) Utilization: a United States Experience Over the Last Decade. Clin Cardiol. 2016;39(2):63–71. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

