Pump-Less, Recirculating Organ-on-Chip (rOoC) Platform to Model the Metabolic Crosstalk between Islets and Liver
- PMID: 38221504
- PMCID: PMC11468483
- DOI: 10.1002/adhm.202303785
Pump-Less, Recirculating Organ-on-Chip (rOoC) Platform to Model the Metabolic Crosstalk between Islets and Liver
Abstract
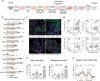
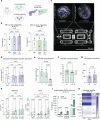
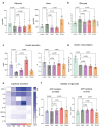
Type 2 diabetes mellitus (T2DM), obesity, and metabolic dysfunction-associated steatotic liver disease (MASLD) are epidemiologically correlated disorders with a worldwide growing prevalence. While the mechanisms leading to the onset and development of these conditions are not fully understood, predictive tissue representations for studying the coordinated interactions between central organs that regulate energy metabolism, particularly the liver and pancreatic islets, are needed. Here, a dual pump-less recirculating organ-on-chip platform that combines human pluripotent stem cell (sc)-derived sc-liver and sc-islet organoids is presented. The platform reproduces key aspects of the metabolic cross-talk between both organs, including glucose levels and selected hormones, and supports the viability and functionality of both sc-islet and sc-liver organoids while preserving a reduced release of pro-inflammatory cytokines. In a model of metabolic disruption in response to treatment with high lipids and fructose, sc-liver organoids exhibit hallmarks of steatosis and insulin resistance, while sc-islets produce pro-inflammatory cytokines on-chip. Finally, the platform reproduces known effects of anti-diabetic drugs on-chip. Taken together, the platform provides a basis for functional studies of obesity, T2DM, and MASLD on-chip, as well as for testing potential therapeutic interventions.
Keywords: Type 2 diabetes (T2DM); drug testing; energy metabolism; metabolic dysfunction‐associated steatotic liver disease (MASLD); obesity; organ‐on‐chip; sc‐islet organoids; sc‐liver organoids.
© 2024 The Authors. Advanced Healthcare Materials published by Wiley‐VCH GmbH.
Conflict of interest statement
A.A., M.B., and S.J.K.K. have applied for a patent covering the main principle of fluid actuation and application and are planning to commercialize the technology.
Figures




References
-
- Rinella M. E., Lazarus J. V., Ratziu V., Francque S. M., Sanyal A. J., Kanwal F., Romero D., Abdelmalek M. F., Anstee Q. M., Arab J. P., Arrese M., Bataller R., Beuers U., Boursier J., Bugianesi E., Byrne C. D., Castro Narro G. E., Chowdhury A., Cortez‐Pinto H., Cryer D. R., Cusi K., El‐Kassas M., Klein S., Eskridge W., Fan J., Gawrieh S., Guy C. D., Harrison S. A., Kim S. U., Koot B. G., et al., J. Hepatol. 2023, 79, 1542. - PubMed
-
- Magliano D. J., Boyko E. J., IDF Diabetes Atlas, 10th ed., International Diabetes Federation, Brussels: 2021. - PubMed
-
- Cotter T. G., Rinella M., Gastroenterology 2020, 158, 1851. - PubMed
-
- Lobstein T., Brinsden H., Neveux M., 2022. https://policycommons.net/artifacts/2266990/world_obesity_atlas_2022_web....
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

