MicroRNA-146a-loaded magnesium silicate nanospheres promote bone regeneration in an inflammatory microenvironment
- PMID: 38221522
- PMCID: PMC10788347
- DOI: 10.1038/s41413-023-00299-0
MicroRNA-146a-loaded magnesium silicate nanospheres promote bone regeneration in an inflammatory microenvironment
Abstract
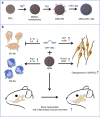
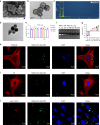
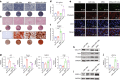
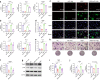
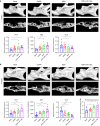
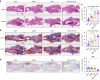
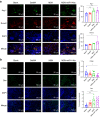
Reconstruction of irregular oral-maxillofacial bone defects with an inflammatory microenvironment remains a challenge, as chronic local inflammation can largely impair bone healing. Here, we used magnesium silicate nanospheres (MSNs) to load microRNA-146a-5p (miR-146a) to fabricate a nanobiomaterial, MSN+miR-146a, which showed synergistic promoting effects on the osteogenic differentiation of human dental pulp stem cells (hDPSCs). In addition, miR-146a exhibited an anti-inflammatory effect on mouse bone marrow-derived macrophages (BMMs) under lipopolysaccharide (LPS) stimulation by inhibiting the NF-κB pathway via targeting tumor necrosis factor receptor-associated factor 6 (TRAF6), and MSNs could simultaneously promote M2 polarization of BMMs. MiR-146a was also found to inhibit osteoclast formation. Finally, the dual osteogenic-promoting and immunoregulatory effects of MSN+miR-146a were further validated in a stimulated infected mouse mandibular bone defect model via delivery by a photocuring hydrogel. Collectively, the MSN+miR-146a complex revealed good potential in treating inflammatory irregular oral-maxillofacial bone defects.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Orthosilicic acid, Si(OH)4, stimulates osteoblast differentiation in vitro by upregulating miR-146a to antagonize NF-κB activation.Acta Biomater. 2016 Jul 15;39:192-202. doi: 10.1016/j.actbio.2016.05.007. Epub 2016 May 6. Acta Biomater. 2016. PMID: 27163405
-
miR-146a-5p promotes epithelium regeneration against LPS-induced inflammatory injury via targeting TAB1/TAK1/NF-κB signaling pathway.Int J Biol Macromol. 2022 Nov 30;221:1031-1040. doi: 10.1016/j.ijbiomac.2022.09.056. Epub 2022 Sep 10. Int J Biol Macromol. 2022. PMID: 36096257
-
miR-146a-5p Attenuates Allergic Airway Inflammation by Inhibiting the NLRP3 Inflammasome Activation in Macrophages.Int Arch Allergy Immunol. 2022;183(9):919-930. doi: 10.1159/000524718. Epub 2022 Jun 3. Int Arch Allergy Immunol. 2022. PMID: 35660690
-
Exosomes Derived from Human Umbilical Cord Mesenchymal Stem Cells Alleviate Diffuse Alveolar Hemorrhage Associated with Systemic Lupus Erythematosus in Mice by Promoting M2 Macrophage Polarization via the microRNA-146a-5p/NOTCH1 Axis.Immunol Invest. 2022 Oct;51(7):1975-1993. doi: 10.1080/08820139.2022.2090261. Epub 2022 Jun 20. Immunol Invest. 2022. PMID: 35723582
-
Exploring microRNAs in craniofacial regenerative medicine.Biochem Soc Trans. 2023 Apr 26;51(2):841-854. doi: 10.1042/BST20221448. Biochem Soc Trans. 2023. PMID: 37073783 Free PMC article. Review.
Cited by
-
Narirutin mitigates inflammatory arthritis and osteoporosis through modulating macrophage phenotype and osteoclastogenesis.J Orthop Translat. 2025 Aug 8;54:115-130. doi: 10.1016/j.jot.2025.07.008. eCollection 2025 Sep. J Orthop Translat. 2025. PMID: 40822517 Free PMC article.
-
Biomaterial-Based Nucleic Acid Delivery Systems for In Situ Tissue Engineering and Regenerative Medicine.Int J Mol Sci. 2025 Jul 30;26(15):7384. doi: 10.3390/ijms26157384. Int J Mol Sci. 2025. PMID: 40806513 Free PMC article. Review.
-
Metal-phenolic network biointerface-mediated cell regulation for bone tissue regeneration.Mater Today Bio. 2024 Dec 12;30:101400. doi: 10.1016/j.mtbio.2024.101400. eCollection 2025 Feb. Mater Today Bio. 2024. PMID: 39759849 Free PMC article. Review.
-
Research progress on mesenchymal stem cell‑derived exosomes in the treatment of osteoporosis induced by knee osteoarthritis (Review).Int J Mol Med. 2025 Oct;56(4):160. doi: 10.3892/ijmm.2025.5601. Epub 2025 Aug 1. Int J Mol Med. 2025. PMID: 40747674 Free PMC article. Review.
-
Serum miR-146a as a diagnostic and predictive biomarker for osteoporosis and bisphosphonate treatment efficacy.Am J Transl Res. 2025 Jul 15;17(7):5320-5331. doi: 10.62347/JRIQ2431. eCollection 2025. Am J Transl Res. 2025. PMID: 40821048 Free PMC article.
References
-
- Candotto V, et al. Complication in third molar extractions. J. Biol. Regul. Homeost. Agents. 2019;33:169–172. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

