Mucosal neuroimmune mechanisms in gastro-oesophageal reflux disease (GORD) pathogenesis
- PMID: 38221552
- PMCID: PMC10904498
- DOI: 10.1007/s00535-023-02065-9
Mucosal neuroimmune mechanisms in gastro-oesophageal reflux disease (GORD) pathogenesis
Abstract
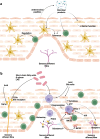
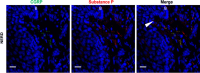
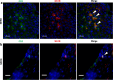
Gastro-oesophageal reflux disease (GORD) is a chronic condition characterised by visceral pain in the distal oesophagus. The current first-line treatment for GORD is proton pump inhibitors (PPIs), however, PPIs are ineffective in a large cohort of patients and long-term use may have adverse effects. Emerging evidence suggests that nerve fibre number and location are likely to play interrelated roles in nociception in the oesophagus of GORD patients. Simultaneously, alterations in cells of the oesophageal mucosa, namely epithelial cells, mast cells, dendritic cells, and T lymphocytes, have been a focus of GORD research for several years. The oesophagus of GORD patients exhibits both macro- and micro-inflammation as a response to chronic acidic reflux at the epithelium. In other conditions of the GI tract, such as IBS and IBD, well-characterised bidirectional processes between immune cells and mucosal nerve fibres contribute to pathogenesis and symptom generation. Sensory alterations in these conditions such as nerve fibre outgrowth and hypersensitivity can be driven by inflammatory processes, which promote visceral pain signalling. This review will examine what is currently known of the molecular pathways linking inflammation and sensory perception leading to the development of GORD symptoms and explore potentially relevant mechanisms in other GI regions which may indicate new areas in GORD research.
Keywords: Gastro-oesophageal reflux disease; Mucosal immunity; Neuroimmune.
© 2024. The Author(s).
Conflict of interest statement
T.L. received an unrestricted, investigator-initiated research grant from Reckitt Benckiser UK. M.P. has no conflicts of interest to disclose.
Figures
Similar articles
-
Pharmacological management of gastro-oesophageal reflux disease.Drugs. 1995 May;49(5):695-710. doi: 10.2165/00003495-199549050-00005. Drugs. 1995. PMID: 7601011 Review.
-
The role of proton pump inhibitors in gastro-oesophageal reflux disease.Drugs. 2004;64(3):277-95. doi: 10.2165/00003495-200464030-00004. Drugs. 2004. PMID: 14871170 Review.
-
Diagnostic value of potent acid inhibition in gastro-oesophageal reflux disease.Drugs. 2005;65 Suppl 1:35-42. doi: 10.2165/00003495-200565001-00006. Drugs. 2005. PMID: 16335856 Review.
-
Progress with novel pharmacological strategies for gastro-oesophageal reflux disease.Drugs. 2004;64(4):347-61. doi: 10.2165/00003495-200464040-00001. Drugs. 2004. PMID: 14969571 Review.
-
GORD and Barrett's oesophagus after bariatric procedures: multicentre prospective study.Br J Surg. 2021 Dec 1;108(12):1498-1505. doi: 10.1093/bjs/znab330. Br J Surg. 2021. PMID: 34738106
Cited by
-
A scientometrics analysis and visualization of refractory gastroesophageal reflux disease.Front Pharmacol. 2024 Jul 30;15:1393526. doi: 10.3389/fphar.2024.1393526. eCollection 2024. Front Pharmacol. 2024. PMID: 39139634 Free PMC article.
-
The oesophagus as an immune organ.Nat Rev Gastroenterol Hepatol. 2025 Jun 18. doi: 10.1038/s41575-025-01086-4. Online ahead of print. Nat Rev Gastroenterol Hepatol. 2025. PMID: 40533621 Review.
-
Multidimensional mechanisms and therapies underlying gastroesophageal reflux disease: focus on immunity, signaling pathways, and the microbiota-gut-brain axis.Front Immunol. 2025 Jul 18;16:1629944. doi: 10.3389/fimmu.2025.1629944. eCollection 2025. Front Immunol. 2025. PMID: 40755761 Free PMC article. Review.
-
Clinical characteristics and risk factors of esophageal reflux hypersensitivity: A multicenter study.World J Gastroenterol. 2025 May 7;31(17):105281. doi: 10.3748/wjg.v31.i17.105281. World J Gastroenterol. 2025. PMID: 40521267 Free PMC article.
References
-
- Weijenborg PW, Smout AJ, Verseijden C, et al. Hypersensitivity to acid is associated with impaired esophageal mucosal integrity in patients with gastroesophageal reflux disease with and without esophagitis. Am J Physiol Gastrointest Liver Physiol. 2014;307:G323–G329. doi: 10.1152/ajpgi.00345.2013. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

