A systematic review for the evidence of recommendations and guidelines in hybrid nuclear cardiovascular imaging
- PMID: 38221570
- PMCID: PMC11178580
- DOI: 10.1007/s00259-024-06597-x
A systematic review for the evidence of recommendations and guidelines in hybrid nuclear cardiovascular imaging
Abstract
Objectives: This study aimed to evaluate the level of evidence of expert recommendations and guidelines for clinical indications and procedurals in hybrid nuclear cardiovascular imaging.
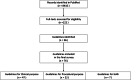
Methods: From inception to August 2023, a PubMed literature analysis of the latest version of guidelines for clinical hybrid cardiovascular imaging techniques including SPECT(/CT), PET(/CT), and PET(/MRI) was performed in two categories: (1) for clinical indications for all-in primary diagnosis; subgroup in prognosis and therapy evaluation; and for (2) imaging procedurals. We surveyed to what degree these followed a standard methodology to collect the data and provide levels of evidence, and for which topic systematic review evidence was executed.
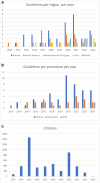
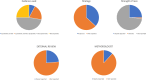
Results: A total of 76 guidelines, published between 2013 and 2023, were included. The evidence of guidelines was based on systematic reviews in 7.9% of cases, non-systematic reviews in 47.4% of cases, a mix of systematic and non-systematic reviews in 19.7%, and 25% of guidelines did not report any evidence. Search strategy was reported in 36.8% of cases. Strengths of recommendation were clearly reported in 25% of guidelines. The notion of external review was explicitly reported in 23.7% of cases. Finally, the support of a methodologist was reported in 11.8% of the included guidelines.
Conclusion: The use of evidence procedures for developing for evidence-based cardiovascular hybrid imaging recommendations and guidelines is currently suboptimal, highlighting the need for more standardized methodological procedures.
Keywords: Cardiovascular guidelines; Evidence-based practice; Hybrid imaging; Positron emission tomography; Recommendations.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
The value of FDG positron emission tomography/computerised tomography (PET/CT) in pre-operative staging of colorectal cancer: a systematic review and economic evaluation.Health Technol Assess. 2011 Sep;15(35):1-192, iii-iv. doi: 10.3310/hta15350. Health Technol Assess. 2011. PMID: 21958472 Free PMC article.
-
Positron emission tomography/computerised tomography imaging in detecting and managing recurrent cervical cancer: systematic review of evidence, elicitation of subjective probabilities and economic modelling.Health Technol Assess. 2013 Mar;17(12):1-323. doi: 10.3310/hta17120. Health Technol Assess. 2013. PMID: 23537558 Free PMC article.
-
123I-MIBG scintigraphy and 18F-FDG-PET imaging for diagnosing neuroblastoma.Cochrane Database Syst Rev. 2015 Sep 29;2015(9):CD009263. doi: 10.1002/14651858.CD009263.pub2. Cochrane Database Syst Rev. 2015. PMID: 26417712 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Health professionals' experience of teamwork education in acute hospital settings: a systematic review of qualitative literature.JBI Database System Rev Implement Rep. 2016 Apr;14(4):96-137. doi: 10.11124/JBISRIR-2016-1843. JBI Database System Rev Implement Rep. 2016. PMID: 27532314
Cited by
-
Splenic Embolism in Infective Endocarditis: A Systematic Review of the Literature with an Emphasis on Radiological and Histopathological Diagnoses.Trop Med Infect Dis. 2024 Apr 12;9(4):83. doi: 10.3390/tropicalmed9040083. Trop Med Infect Dis. 2024. PMID: 38668544 Free PMC article. Review.
References
-
- Carneiro AV. Methodological appraisal of guidelines. The AGREE instrument. Rev Port Cardiol. 2004;23:447–456. - PubMed
-
- Qaseem A, Kansagara D, Lin JS, Mustafa RA, Wilt TJ, Clinical Guidelines Committee of the American College of P et al. The Development of Clinical Guidelines and Guidance Statements by the Clinical Guidelines Committee of the American College of Physicians: Update of Methods. Ann Intern Med. 2019;170:863–70. doi: 10.7326/M18-3290. - DOI - PubMed
-
- WHO Handbook for Guideline development. Extra info: WHO handbook for guideline development – 2nd ed. 1. Guidelines as Topic – standards. 2. Review. 3. Meta-Analysis. 4. Peer Review. 5. Evidence-Based Medicine. 6. World Health Organization. I. World Health Organization. 2014
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources