Real-World Treatment and Care Patterns in Patients With Rheumatoid Arthritis Initiating First-Line Tumor Necrosis Factor Inhibitor Therapy in the United States
- PMID: 38221639
- PMCID: PMC11016569
- DOI: 10.1002/acr2.11646
Real-World Treatment and Care Patterns in Patients With Rheumatoid Arthritis Initiating First-Line Tumor Necrosis Factor Inhibitor Therapy in the United States
Abstract
Objective: Treatment guidelines for rheumatoid arthritis (RA) recommend targeting low disease activity or remission and switching therapies for patients not reaching those targets. We evaluated real-world use of disease activity measures, treatment discontinuation, and switching patterns among patients with RA initiating a first-line tumor necrosis factor inhibitor (TNFi).
Methods: Data from adult patients with RA initiating a first-line TNFi were collected from the American Rheumatology Network (January 2014-August 2021). The proportion of patients with recorded disease activity scores (Clinical Disease Activity Index [CDAI] or Routine Assessment of Patient Index Data 3 [RAPID3]) at TNFi initiation was assessed. Among patients with moderate or severe RA at TNFi initiation, reasons for discontinuation and subsequent advanced therapy were evaluated.
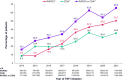
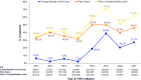
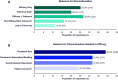
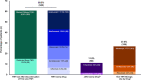
Results: Among TNFi initiators (n = 15,182), 44.8% recorded a CDAI/RAPID3 score at treatment initiation; of those who did not, 47.0% had recorded a tender and/or swollen joint count or pain score. Among patients with moderate or severe RA (n = 1,651), 52% discontinued their initial TNFi during follow-up, of which 15%, 46%, 28%, and 12% initiated the same TNFi, another TNFi, a non-TNFi biologic, or a Janus kinase inhibitor, respectively. The proportion of patients restarting the same TNFi or initiating another TNFi varied according to TNFi discontinuation reason.
Conclusion: In clinical practice, over half of patients with RA initiating a first-line TNFi did not have baseline disease activity assessments. Many patients cycled through TNFi despite citing lack of efficacy as the most common reason for discontinuation. Consistent, objective monitoring of treatment response and timely switch to effective therapy is needed in patients with RA.
© 2024 AbbVie Inc and The Authors. ACR Open Rheumatology published by Wiley Periodicals LLC on behalf of American College of Rheumatology.
Figures
Similar articles
-
Cycling of tumor necrosis factor inhibitors versus switching to different mechanism of action therapy in rheumatoid arthritis patients with inadequate response to tumor necrosis factor inhibitors: a Bayesian network meta-analysis.Ther Adv Musculoskelet Dis. 2021 Mar 29;13:1759720X211002682. doi: 10.1177/1759720X211002682. eCollection 2021. Ther Adv Musculoskelet Dis. 2021. PMID: 33854570 Free PMC article.
-
Effect of Anticitrullinated Protein Antibody Status on Response to Abatacept or Antitumor Necrosis Factor-α Therapy in Patients with Rheumatoid Arthritis: A US National Observational Study.J Rheumatol. 2018 Jan;45(1):32-39. doi: 10.3899/jrheum.170007. Epub 2017 Nov 1. J Rheumatol. 2018. PMID: 29093151
-
Predictors of Treatment Change Among Patients with Rheumatoid Arthritis Treated with TNF Inhibitors as First-Line Biologic Agent in the USA: A Cohort Study from Longitudinal Electronic Health Records.BioDrugs. 2022 Jul;36(4):521-535. doi: 10.1007/s40259-022-00542-w. Epub 2022 Jun 30. BioDrugs. 2022. PMID: 35771381
-
Comparative effectiveness of TNF inhibitor vs IL-6 receptor inhibitor as monotherapy or combination therapy with methotrexate in biologic-experienced patients with rheumatoid arthritis: An analysis from the CorEvitas RA Registry.Clin Rheumatol. 2023 Aug;42(8):2037-2051. doi: 10.1007/s10067-023-06588-7. Epub 2023 Apr 15. Clin Rheumatol. 2023. PMID: 37060528 Free PMC article.
-
Sarilumab for Previously-Treated Moderate or Severe Rheumatoid Arthritis: An Evidence Review Group Perspective of a NICE Single Technology Appraisal.Pharmacoeconomics. 2018 Dec;36(12):1427-1437. doi: 10.1007/s40273-018-0677-7. Pharmacoeconomics. 2018. PMID: 29882210 Review.
References
-
- Fraenkel L, Bathon JM, England BR, et al. 2021 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol 2021;73(7):1108–1123. - PubMed
-
- Smolen JS, Landewe RBM, Bijlsma JWJ, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease‐modifying antirheumatic drugs: 2019 update. Ann Rheum Dis 2020;79(6):685–699. - PubMed
-
- Singh JA, Saag KG, Bridges SL, Jr , et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol 2016;68(1):1–26. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

