Ex vivo expansion of human hematopoietic stem cells and clinical applications
- PMID: 38221718
- PMCID: PMC10921004
- DOI: 10.1111/cas.16066
Ex vivo expansion of human hematopoietic stem cells and clinical applications
Abstract
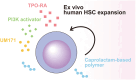
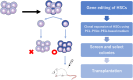
Hematopoietic stem cells (HSCs) are a rare population of cells found in the bone marrow that play a critical role in lifelong hematopoiesis and the reconstitution of the hematopoietic system after hematopoietic stem cell transplantation. Hematopoietic stem cell transplantation remains the only curative treatment for patients with refractory hematologic disorders, and umbilical cord blood (CB) serves as an alternative stem cell source due to its several advantageous characteristics, including human leukocyte antigen flexibility and reduced donor burden. However, CB also has the disadvantage of containing a small number of cells, resulting in limited donor selection and a longer time for engraftment. Therefore, the development of techniques to expand HSCs ex vivo, particularly umbilical CB, is a goal in hematology. While various combinations of cytokines were once the mainstream approach, these protocols had limited expansion rates and did not lead to clinical application. However, in recent years, the development of a technique in which small molecules are added to cytokines has enabled the stable, long-term ex vivo expansion of human HSCs. Clinical trials of expanded umbilical CB using these techniques have been undertaken and have confirmed their efficacy and safety. In addition, we have successfully developed a recombinant-cytokine-free and albumin-free culture system for the long-term expansion of human HSCs. This approach could offer the potential for more selective expansion of human HSCs compared to previous protocols. This review discusses ex vivo culture protocols for expanding human HSCs and presents the results of clinical trials using these techniques, along with future perspectives.
Keywords: chemical; clinical trial; ex vivo expansion; human hematopoietic stem cell; polymer.
© 2024 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
M.S. and S.Y. are cofounders and shareholders in Celaid Therapeutics. S.Y. has received research funding from BASF. The other authors declare that they have no conflicts of interest.
Figures
References
-
- Gluckman E, Broxmeyer HE, Auerbach AD, et al. Hematopoietic reconstitution in a patient with Fanconi's anemia by means of umbilical‐cord blood from an HLA‐identical sibling. N Engl J Med. 1989;321:1174‐1178. - PubMed
-
- Williams DE, Eisenman J, Baird A, et al. Identification of a ligand for the c‐kit proto‐oncogene. Cell. 1990;63:167‐174. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

