A Systematic Literature Review to Identify Diagnostic Gaps in Managing Immunocompromised Patients With Cancer and Suspected Infection
- PMID: 38221981
- PMCID: PMC10787371
- DOI: 10.1093/ofid/ofad616
A Systematic Literature Review to Identify Diagnostic Gaps in Managing Immunocompromised Patients With Cancer and Suspected Infection
Abstract
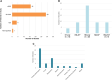
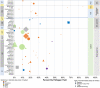
Patients with cancer are increasingly vulnerable to infections, which may be more severe than in the general population. Improvements in rapid and timely diagnosis to optimize management are needed. We conducted a systematic literature review to determine the unmet need in diagnosing acute infections in immunocompromised patients with cancer and identified 50 eligible studies from 5188 records between 1 January 2012 and 23 June 2022. There was considerable heterogeneity in study designs and parameters, laboratory methods and definitions, and assessed outcomes, with limited evaluation of diagnostic impact on clinical outcomes. Culture remains the primary diagnostic strategy. Fewer studies employing molecular technologies exist, but emerging literature suggests that pathogen-agnostic molecular tests may add to the diagnostic armamentarium. Well-designed clinical studies using standardized methodologies are needed to better evaluate performance characteristics and clinical and economic impacts of emerging diagnostic techniques to improve patient outcomes.
Keywords: cancer; diagnostic; immunocompromised; infection; metagenomic sequencing.
© The Author(s) 2023. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. R. T. and J. A.H. are compensated members of the Scientific Advisory Board for Karius. S. Y. P. is employed by Karius. K. J. reports no conflicts.
Figures



References
-
- Wang XJ, Lopez SE, Chan A. Economic burden of chemotherapy-induced febrile neutropenia in patients with lymphoma: a systematic review. Crit Rev Oncol Hematol 2015; 94:201–12. - PubMed
-
- Weycker D, Li X, Edelsberg J, et al. Risk and consequences of chemotherapy-induced febrile neutropenia in patients with metastatic solid tumors. J Oncol Pract 2015; 11:47–54. - PubMed
-
- Jordana-Lluch E, Rivaya B, Marcó C, et al. Molecular diagnosis of bloodstream infections in onco-haematology patients with PCR/ESI-MS technology. J Infect 2017; 74:187–94. - PubMed
-
- Wilson B, Zitella L, Erb C, Foster J, Peterson M, Wood S. Prevention of infection: a systematic review of evidence-based practice interventions for management in patients with cancer. Clin J Oncol Nurs 2018; 22:157–68. - PubMed

