Similarities and Differences of Vascular Calcification in Diabetes and Chronic Kidney Disease
- PMID: 38222032
- PMCID: PMC10788067
- DOI: 10.2147/DMSO.S438618
Similarities and Differences of Vascular Calcification in Diabetes and Chronic Kidney Disease
Abstract
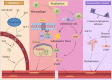
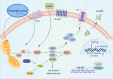
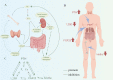
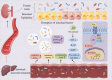
Presently, the mechanism of occurrence and development of vascular calcification (VC) is not fully understood; a range of evidence suggests a positive association between diabetes mellitus (DM) and VC. Furthermore, the increasing burden of central vascular disease in patients with chronic kidney disease (CKD) may be due, at least in part, to VC. In this review, we will review recent advances in the mechanisms of VC in the context of CKD and diabetes. The study further unveiled that VC is induced through the stimulation of pro-inflammatory factors, which in turn impairs endothelial function and triggers similar mechanisms in both disease contexts. Notably, hyperglycemia was identified as the distinctive mechanism driving calcification in DM. Conversely, in CKD, calcification is facilitated by mechanisms including mineral metabolism imbalance and the presence of uremic toxins. Additionally, we underscore the significance of investigating vascular alterations and newly identified molecular pathways as potential avenues for therapeutic intervention.
Keywords: chronic kidney disease; diabetes mellitus; vascular calcification.
© 2024 Wang et al.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Vascular Calcification in Chronic Kidney Disease: Diversity in the Vessel Wall.Biomedicines. 2021 Apr 8;9(4):404. doi: 10.3390/biomedicines9040404. Biomedicines. 2021. PMID: 33917965 Free PMC article. Review.
-
Contribution of Gut Microbiota-Derived Uremic Toxins to the Cardiovascular System Mineralization.Toxins (Basel). 2021 Apr 10;13(4):274. doi: 10.3390/toxins13040274. Toxins (Basel). 2021. PMID: 33920096 Free PMC article. Review.
-
Vascular calcification in long-term kidney transplantation.Nephrology (Carlton). 2014 Apr;19(4):251-6. doi: 10.1111/nep.12210. Nephrology (Carlton). 2014. PMID: 24447254
-
Vascular calcification in CKD: New insights into its mechanisms.J Cell Physiol. 2023 Jun;238(6):1160-1182. doi: 10.1002/jcp.31021. Epub 2023 Jun 3. J Cell Physiol. 2023. PMID: 37269534 Review.
-
The interplay between mineral metabolism, vascular calcification and inflammation in Chronic Kidney Disease (CKD): challenging old concepts with new facts.Aging (Albany NY). 2019 Jun 26;11(12):4274-4299. doi: 10.18632/aging.102046. Aging (Albany NY). 2019. PMID: 31241466 Free PMC article. Review.
Cited by
-
Elevated glucose levels increase vascular calcification risk by disrupting extracellular pyrophosphate metabolism.Cardiovasc Diabetol. 2024 Nov 11;23(1):405. doi: 10.1186/s12933-024-02502-w. Cardiovasc Diabetol. 2024. PMID: 39529124 Free PMC article.
-
Use of CT-derived non-cardiovascular calcification marker for predicting cardiovascular events among diabetic older adults: the multi-ethnic study of atherosclerosis.Eur Radiol. 2025 Aug 9. doi: 10.1007/s00330-025-11778-9. Online ahead of print. Eur Radiol. 2025. PMID: 40782222
References
Publication types
LinkOut - more resources
Full Text Sources

