MOFganic Chemistry: Challenges and Opportunities for Metal-Organic Frameworks in Synthetic Organic Chemistry
- PMID: 38222037
- PMCID: PMC10785605
- DOI: 10.1021/acs.chemmater.3c00741
MOFganic Chemistry: Challenges and Opportunities for Metal-Organic Frameworks in Synthetic Organic Chemistry
Abstract
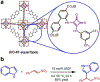
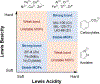
Metal-organic frameworks (MOFs) are porous, crystalline solids constructed from organic linkers and inorganic nodes that have been widely studied for applications in gas storage, chemical separations, and drug delivery. Owing to their highly modular structures and tunable pore environments, we propose that MOFs have significant untapped potential as catalysts and reagents relevant to the synthesis of next-generation therapeutics. Herein, we outline the properties of MOFs that make them promising for applications in synthetic organic chemistry, including new reactivity and selectivity, enhanced robustness, and user-friendly preparation. In addition, we outline the challenges facing the field and propose new directions to maximize the utility of MOFs for drug synthesis. This perspective aims to bring together the organic and MOF communities to develop new heterogeneous platforms capable of achieving synthetic transformations that cannot be replicated by homogeneous systems.
Figures
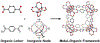


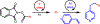



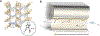
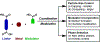
References
-
- Fechete I; Wang Y; Védrine JC The Past, Present and Future of Heterogeneous Catalysis. Catalysis Today 2012, 189 (1), 2–27. 10.1016/j.cattod.2012.04.003. - DOI
-
- Gladysz JA Recoverable Catalysts. Ultimate Goals, Criteria of Evaluation, and the Green Chemistry Interface. Pure Appl. Chem 2001, 73 (8), 1319–1324. 10.1351/pac200173081319. - DOI
-
- Bryan MC; Dunn PJ; Entwistle D; Gallou F; Koenig SG; Hayler JD; Hickey MR; Hughes S; Kopach ME; Moine G; Richardson P; Roschangar F; Steven A; Weiberth FJ Key Green Chemistry Research Areas from a Pharmaceutical Manufacturers’ Perspective Revisited. Green Chem. 2018, 20 (22), 5082–5103. 10.1039/C8GC01276H. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
