Effects of a Remote Multimodal Intervention Involving Diet, Walking Program, and Breathing Exercise on Quality of Life Among Newly Diagnosed People with Multiple Sclerosis: A Quasi-Experimental Non-Inferiority Pilot Study
- PMID: 38222092
- PMCID: PMC10787513
- DOI: 10.2147/DNND.S441738
Effects of a Remote Multimodal Intervention Involving Diet, Walking Program, and Breathing Exercise on Quality of Life Among Newly Diagnosed People with Multiple Sclerosis: A Quasi-Experimental Non-Inferiority Pilot Study
Abstract
Background: Interventions involving diet, physical activity, and breathing exercises are shown to be beneficial in managing both fatigue and quality of life (QoL) related to MS; however, the impact of such interventions among people newly diagnosed with clinically isolated syndrome (CIS) or relapsing-remitting multiple sclerosis (RRMS) who decline disease-modifying therapies (DMTs) is unknown.
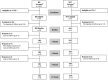
Methods: A 12-month prospective quasi-experimental non-inferiority trial recruited people newly diagnosed with CIS or RRMS who voluntarily declined DMTs (health behavior group; HB, n = 29) or followed standard of care (SOC, n = 15). Participants in the HB group were remotely coached on the study diet, moderate-intensity walking, and breathing exercises. All participants completed questionnaires validated to assess MS symptoms, including perceived mental and physical QoL (MSQOL54); fatigue (Fatigue Severity Scale, FSS; and Modified Fatigue Impact Scale, MFIS); mood (Hospital Anxiety and Depression Scale, HADS); and cognitive function (Perceived Deficits Questionnaire, PDQ).
Results: During the 12 months, the HB group experienced improvement in scores for mental QoL (MSQOL54 - Mental, 0.24, 95% CI 0.01, 0.47; p = 0.04), fatigue (Total MFIS, -7.26, 95% CI -13.3,-1.18; p = 0.02), and perceived cognitive function (Total PDQ, PDQ-Attention, PDQ-Promemory, and PDQ-Planning, p ≤ 0.03 for all). A between-group difference was observed only for PDQ-Planning (p = 0.048). Non-inferiority analysis revealed that the 12-month changes in means for the HB group were not worse than those for the SOC group with respect to fatigue (FSS, p = 0.02), mood (HDS-Anxiety, p = 0.02; HADS-Depression, p < 0.0001), physical QoL (MSQOL54 - Physical, p = 0.02), or cognitive dysfunction (Total PDQ, p = 0.01).
Conclusion: The multimodal lifestyle intervention for individuals newly diagnosed with CIS or RRMS, who voluntarily decline DMTs, did not yield patient-reported outcomes worse than those observed in the SOC group regarding perceived mental quality of life, mood, fatigue, and cognitive function.
Trial registration: clinicaltrials.gov identifier: NCT04009005.
Keywords: mindfulness-based breathing; modified paleolithic diet; multiple sclerosis; physical activity; quasi-experimental.
© 2024 Saxby et al.
Conflict of interest statement
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Conflict of Interest Disclosures: Dr Terry Wahls personally follows and promotes the Wahls™ diet. She has equity interest in the following companies: Terry Wahls LLC; TZ Press LLC; The Wahls Institute, PLC; FBB Biomed Inc.; Levels Health Inc., and the website http://www.terrywahls.com. She also owns the copyright to the books Minding My Mitochondria (2nd Edition) and The Wahls Protocol, The Wahls Protocol Cooking for Life, and the trademarks The Wahls Protocol® and Wahls™ diet, Wahls Paleo™ diet, and Wahls Paleo Plus™ diets. She has completed grant funding from the National Multiple Sclerosis Society for the Dietary Approaches to Treating Multiple Sclerosis Related Fatigue Study. She has financial relationships with Vibrant America LLC, Standard Process Inc., MasterHealth Technologies Inc., Foogal Inc., and the Institute for Functional Medicine Inc. She receives royalty payments from Penguin Random House. Dr Wahls has conflict-of-interest management plans in place with the University of Iowa and the Iowa City Veteran’s Affairs Medical Center. This study received funding from Dr Terry Wahls LLC. The funder had the following involvement with the study: study design, the writing of this article, and the decision to submit it for publication. Also, Dr Tyler Titcomb received Fellowship fund that supported me for this study from the Carter Chapman Shreve Family Foundation, during the conduct of the study. All authors declare no other competing interests.
Figures
Similar articles
-
Feasibility and assessment of self-reported dietary recalls among newly diagnosed multiple sclerosis: a quasi-experimental pilot study.Front Nutr. 2024 Oct 11;11:1369700. doi: 10.3389/fnut.2024.1369700. eCollection 2024. Front Nutr. 2024. PMID: 39464680 Free PMC article.
-
Evaluation of a web-based program for the adoption of wellness behaviors to self-manage fatigue and improve quality of life among people with multiple sclerosis: A randomized waitlist-control trial.Mult Scler Relat Disord. 2023 Sep;77:104858. doi: 10.1016/j.msard.2023.104858. Epub 2023 Jun 27. Mult Scler Relat Disord. 2023. PMID: 37399671 Free PMC article. Clinical Trial.
-
Dietary approaches to treat MS-related fatigue: comparing the modified Paleolithic (Wahls Elimination) and low saturated fat (Swank) diets on perceived fatigue in persons with relapsing-remitting multiple sclerosis: study protocol for a randomized controlled trial.Trials. 2018 Jun 4;19(1):309. doi: 10.1186/s13063-018-2680-x. Trials. 2018. PMID: 29866196 Free PMC article.
-
Behavioural modification interventions for medically unexplained symptoms in primary care: systematic reviews and economic evaluation.Health Technol Assess. 2020 Sep;24(46):1-490. doi: 10.3310/hta24460. Health Technol Assess. 2020. PMID: 32975190 Free PMC article.
-
Palliative care interventions for people with multiple sclerosis.Cochrane Database Syst Rev. 2019 Oct 22;10(10):CD012936. doi: 10.1002/14651858.CD012936.pub2. Cochrane Database Syst Rev. 2019. PMID: 31637711 Free PMC article. Review.
Cited by
-
Feasibility and assessment of self-reported dietary recalls among newly diagnosed multiple sclerosis: a quasi-experimental pilot study.Front Nutr. 2024 Oct 11;11:1369700. doi: 10.3389/fnut.2024.1369700. eCollection 2024. Front Nutr. 2024. PMID: 39464680 Free PMC article.
-
Baseline engagement with healthy lifestyles and their associations with health outcomes in people with multiple sclerosis enrolled in an online multimodal lifestyle course.Eur J Neurol. 2024 Oct;31(10):e16429. doi: 10.1111/ene.16429. Epub 2024 Aug 7. Eur J Neurol. 2024. PMID: 39109838 Free PMC article. Clinical Trial.
References
Publication types
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

