Synthesis, Characterization, Antiglycation Evaluation, Molecular Docking, and ADMET Studies of 4-Thiazolidinone Derivatives
- PMID: 38222574
- PMCID: PMC10785283
- DOI: 10.1021/acsomega.3c08463
Synthesis, Characterization, Antiglycation Evaluation, Molecular Docking, and ADMET Studies of 4-Thiazolidinone Derivatives
Abstract
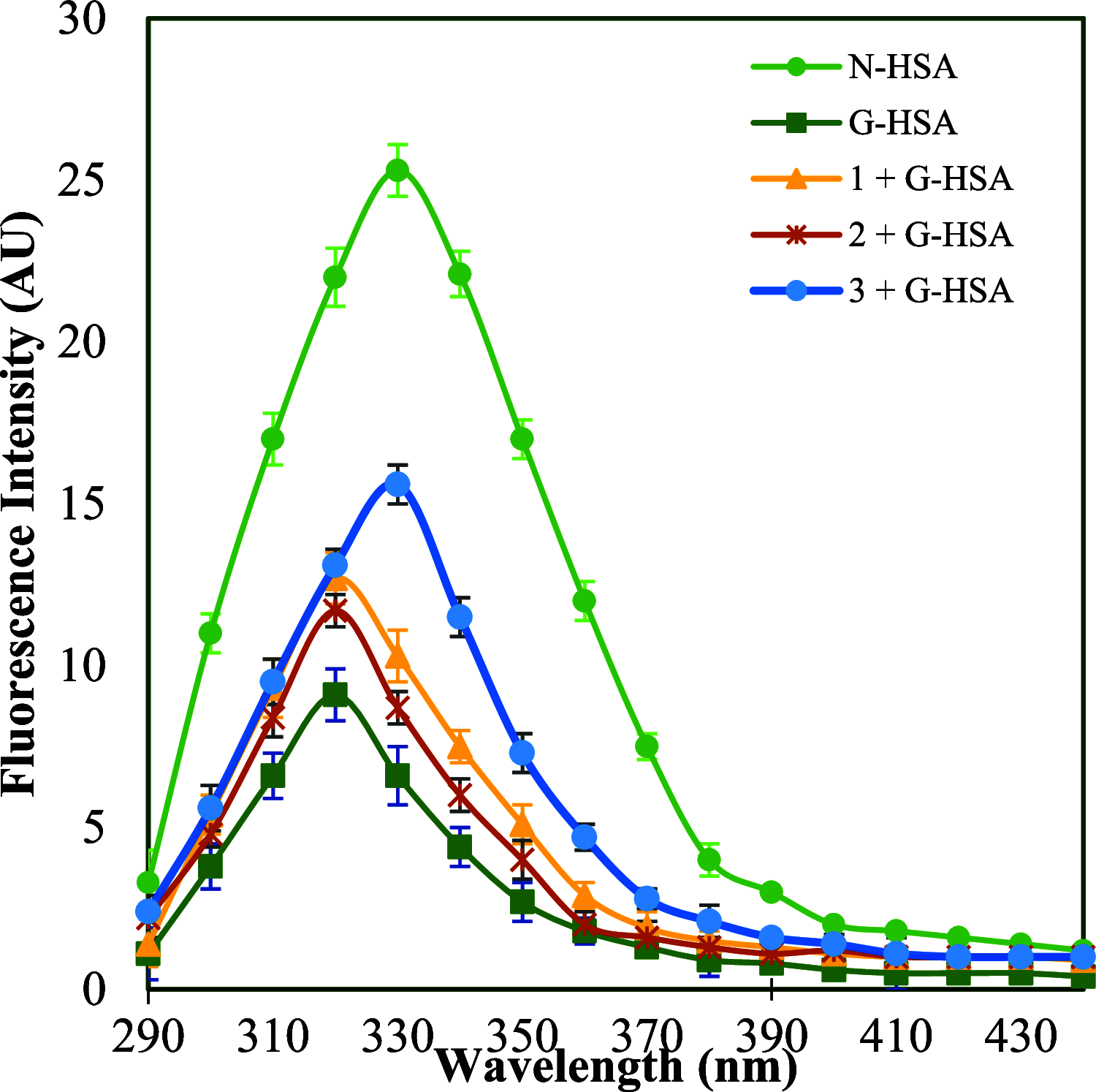
The design and development of new small-molecule glycation inhibitors are essential for preventing various chronic diseases, including diabetes mellitus, immunoinflammation, cardiovascular, and neurodegenerative diseases. 4-Thiazolidinone or thiazolidine-4-one is a well-known heterocyclic compound with the potential to inhibit the formation of advanced glycation end products. In the present work, we report the synthesis and characterization of four new 5-arylidene 3-cyclopropyl-2-(phenylimino)thiazolidin-4-one (1-4) compounds and their human serum albumin glycation inhibitory activity. One of the compounds 5-(2H-1,3-benzodioxol-5-ylmethylidene)-3-cyclopropyl-2-(phenylimino)-1,3-thiazolidin-4-one (3) showed potent inhibition in the synthesis of initial, intermediary, and final products of glycation reactions. Besides, conformational changes in the α-helix and β-sheet (due to hyperglycemia) were also found to be reversed upon the addition of (3). Experimental findings were complemented by computational [molecular docking, ADME/Tox, and density functional theory (DFT)] studies. The docking scores of the compounds were in order 1 > 3 > 2 > 4, indicating the importance of the polar group at the 5-arylidene moiety. The results of ADME/Tox and DFT calculations revealed the safe nature of the compounds with high drug-likeness and stability. Overall, we speculate that the results of this study could provide valuable insights into the biological activity of 4-thiazolidinones.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





Similar articles
-
Antiglycation Activity of Triazole Schiff's Bases Against Fructosemediated Glycation: In Vitro and In Silico Study.Med Chem. 2020;16(4):575-591. doi: 10.2174/1573406415666190212105718. Med Chem. 2020. PMID: 30747076
-
Molecular docking, drug-likeness and DFT study of some modified tetrahydrocurcumins as potential anticancer agents.Saudi Pharm J. 2024 Jan;32(1):101889. doi: 10.1016/j.jsps.2023.101889. Epub 2023 Dec 1. Saudi Pharm J. 2024. PMID: 38090737 Free PMC article.
-
Investigation of the New Inhibitors by Sulfadiazine and Modified Derivatives of α-D-glucopyranoside for White Spot Syndrome Virus Disease of Shrimp by In Silico: Quantum Calculations, Molecular Docking, ADMET and Molecular Dynamics Study.Molecules. 2022 Jun 8;27(12):3694. doi: 10.3390/molecules27123694. Molecules. 2022. PMID: 35744817 Free PMC article.
-
Design, Synthesis, Kinetic Analysis and Pharmacophore-Directed Discovery of 3-Ethylaniline Hybrid Imino-Thiazolidinone as Potential Inhibitor of Carbonic Anhydrase II: An Emerging Biological Target for Treatment of Cancer.Biomolecules. 2022 Nov 16;12(11):1696. doi: 10.3390/biom12111696. Biomolecules. 2022. PMID: 36421710 Free PMC article.
-
Antiglycation therapy: Discovery of promising antiglycation agents for the management of diabetic complications.Pharm Biol. 2016;54(2):198-206. doi: 10.3109/13880209.2015.1028080. Epub 2015 Apr 8. Pharm Biol. 2016. PMID: 25853955 Review.
Cited by
-
Evaluation of In Silico and In Vitro Antidiabetic Properties of 4-(Hydroxysubstituted Arylidene)-2-Phenyloxazol-5(4H)-one Derivatives.ACS Omega. 2025 Feb 18;10(8):8009-8022. doi: 10.1021/acsomega.4c09061. eCollection 2025 Mar 4. ACS Omega. 2025. PMID: 40060839 Free PMC article.
References
-
- Lee B.-W.; Chae H. Y.; Kwon S. J.; Park S. Y.; Ihm J.; Ihm S.-H. RAGE ligands induce apoptotic cell death of pancreatic β-cells via oxidative stress. Int. J. Mol. Med. 2010, 26 (6), 813–818. 10.3892/ijmm_00000529. - DOI - PubMed
- Lin N.; Zhang H.; Su Q. Advanced glycation end-products induce injury to pancreatic beta cells through oxidative stress. Diabetes Metabol. 2012, 38 (3), 250–257. 10.1016/j.diabet.2012.01.003. - DOI - PubMed
-
- Asgharpour Dil F.; Ranjkesh Z.; Goodarzi M. T. A systematic review of antiglycation medicinal plants. Diabetes Metabol. Syndr. 2019, 13 (2), 1225–1229. 10.1016/j.dsx.2019.01.053. - DOI - PubMed
- Abbas G.; Al-Harrasi A. S.; Hussain H.; Hussain J.; Rashid R.; Choudhary M. I. Antiglycation therapy: discovery of promising antiglycation agents for the management of diabetic complications. Pharmaceut. Biol. 2016, 54 (2), 198–206. 10.3109/13880209.2015.1028080. - DOI - PubMed
LinkOut - more resources
Full Text Sources
