pH-Sensitive Acrylic Terpolymers for the Coating of Orally Administered Drugs Used for Colonic Release
- PMID: 38222599
- PMCID: PMC10785650
- DOI: 10.1021/acsomega.3c03437
pH-Sensitive Acrylic Terpolymers for the Coating of Orally Administered Drugs Used for Colonic Release
Abstract
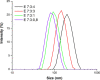
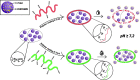
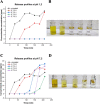
Polymeric coatings are a promising option for the development of delivery systems for orally administered drugs. However, the gastrointestinal conditions to which they are subjected, which include low pH and solubility as well as peristaltic movements, can limit their applications. In this work, different formulations of polymeric coatings were produced using pH-sensitive materials consisting of copolymers of methyl acrylate, methyl methacrylate, and methacrylic acid. The polymers were synthesized by the emulsion polymerization technique, obtaining small average particle sizes (56-190 nm), molecular weights between 200,000 and 400,000 g/mol, and a glass transition temperature above 35 °C, which are suitable for film formation at room temperature. Thus, they were assessed as coatings for hydroxypropyl methylcellulose capsules (HPMC) using the immersion method, showing adequate capacity to protect the capsule at gastric pH (pH 1.2) and dissolve at the simulated intestinal pH (pH= 7.2). In particular, the higher the content of the acidic monomer, the higher the release time of the test molecule contained in the acrylic terpolymer-coated HPMC capsules proposed, which was a curcuminoid derivative due to their bright color and potential medical benefits. In addition, a minimum number of immersions was required for coating the HPMC capsules at high acidic concentrations, which further facilitates the delayed release needed for colonic treatment. However, too high proportions of methacrylic acid may result in cytotoxicity issues. Consequently, a biocompatible formulation containing a proportion of methyl acrylate, methyl methacrylate, and methacrylic acid of 7:3:3 is proposed as the most adequate for colonic release. Thus, by chemically modulating the molar percentages of the acrylic monomers, it was possible to obtain tailored acrylic terpolymer coatings with different characteristics and desired properties in order to modulate the release kinetics of an active substance in a colonic environment.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures







References
-
- Babadi D.; Dadashzadeh S.; Osouli M.; Abbasian Z.; Daryabari M. S.; Sadrai S.; Haeri A. Biopharmaceutical and Pharmacokinetic Aspects of Nanocarrier-Mediated Oral Delivery of Poorly Soluble Drugs. J. Drug Delivery Sci. Technol. 2021, 62, 10232410.1016/j.jddst.2021.102324. - DOI
LinkOut - more resources
Full Text Sources
