Salicylic Acid Conjugate of Telmisartan Inhibits Chikungunya Virus Infection and Inflammation
- PMID: 38222605
- PMCID: PMC10785651
- DOI: 10.1021/acsomega.3c00763
Salicylic Acid Conjugate of Telmisartan Inhibits Chikungunya Virus Infection and Inflammation
Abstract
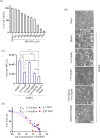
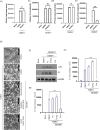
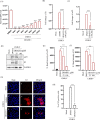
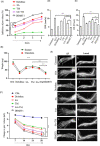
There is no approved antiviral for the management of the Chikungunya virus (CHIKV). To develop an antiviral drug that can manage both CHIKV and arthritis induced by it, an ester conjugate of telmisartan (TM) and salicylic acid (SA) was synthesized (DDABT1). It showed higher potency (IC50 of 14.53 μM) and a good selectivity index [(SI = CC50/IC50) > 33]. On post-treatment of DDABT1, CHIKV infection was inhibited significantly by reducing CPE, viral titer, viral RNA, and viral proteins. Further, the time of addition experiment revealed >95% inhibition up to 4hpi indicating its interference predominantly in the early stages of infection. However, the late stages were also affected. This conjugate of SA and TM was found to increase the antiviral efficacy, and this might be partly attributed to modulating angiotensin II (Ang II) receptor type 1 (AT1). However, DDABT1 might have other modes of action that need further investigation. In addition, the in vivo experiments showed an LD50 of 5000 mg/kg in rats and was found to be more effective than TM, SA, or their combination against acute, subacute, and chronic inflammation/arthritis in vivo. In conclusion, DDABT1 showed remarkable anti-CHIKV properties and the ability to reduce inflammation and arthritis, making it a very good potential drug candidate that needs further experimental validation.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Telmisartan Restricts Chikungunya Virus Infection In Vitro and In Vivo through the AT1/PPAR-γ/MAPKs Pathways.Antimicrob Agents Chemother. 2022 Jan 18;66(1):e0148921. doi: 10.1128/AAC.01489-21. Epub 2021 Nov 8. Antimicrob Agents Chemother. 2022. PMID: 34748384 Free PMC article.
-
Conjugates of ibuprofen inhibit CHIKV infection and inflammation.Mol Divers. 2024 Jun;28(3):1261-1272. doi: 10.1007/s11030-023-10654-2. Epub 2023 Apr 21. Mol Divers. 2024. PMID: 37085737
-
Repurposing potential of rimantadine hydrochloride and development of a promising platinum(II)-rimantadine metallodrug for the treatment of Chikungunya virus infection.Acta Trop. 2022 Mar;227:106300. doi: 10.1016/j.actatropica.2021.106300. Epub 2021 Dec 31. Acta Trop. 2022. PMID: 34979144
-
Current Strategies for Inhibition of Chikungunya Infection.Viruses. 2018 May 3;10(5):235. doi: 10.3390/v10050235. Viruses. 2018. PMID: 29751486 Free PMC article. Review.
-
Prophylactic strategies to control chikungunya virus infection.Virus Genes. 2021 Apr;57(2):133-150. doi: 10.1007/s11262-020-01820-x. Epub 2021 Feb 15. Virus Genes. 2021. PMID: 33590406 Free PMC article. Review.
Cited by
-
Network Pharmacology, Molecular Docking and in vivo-based Analysis on the Effects of the MBZM-N-IBT for Arthritis.Curr Comput Aided Drug Des. 2025;21(2):194-210. doi: 10.2174/0115734099307360240731052835. Curr Comput Aided Drug Des. 2025. PMID: 39108124
-
Drug repurposing targeting COVID-19 3CL protease using molecular docking and machine learning regression approaches.Sci Rep. 2025 May 28;15(1):18722. doi: 10.1038/s41598-025-02773-7. Sci Rep. 2025. PMID: 40436944 Free PMC article.
References
-
- Weaver S. C.; Lecuit M. Chikungunya virus and the global spread of a mosquito-borne disease. N Engl J. Med. 2015, 372 (13), 1231–1239. 10.1056/NEJMra1406035. - DOI - PubMed
- Simon F.; Javelle E.; Cabie A.; Bouquillard E.; Troisgros O.; Gentile G.; Leparc-Goffart I.; Hoen B.; Gandjbakhch F.; Rene-Corail P.; et al. French guidelines for the management of chikungunya (acute and persistent presentations). November 2014. Med. Mal Infect 2015, 45 (7), 243–263. 10.1016/j.medmal.2015.05.007. - DOI - PubMed
-
- Kumar A.; Mishra P.; Chattopadhyay S.; Subudhi B. B. Current Strategies for Inhibition of Chikungunya Infection. Viruses 2018, 10 (5), 235.10.3390/v10050235. - DOI - PMC - PubMed
- Kovacikova K.; van Hemert M. J. Small-Molecule Inhibitors of Chikungunya Virus: Mechanisms of Action and Antiviral Drug Resistance. Antimicrob. Agents Chemother. 2020, 64 (12), e01788-2010.1128/AAC.01788-20. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
