Combined Experimental and Theoretical Insights: Spectroscopic and Molecular Investigation of Polyphenols from Fagonia indica via DFT, UV-vis, and FT-IR Approaches
- PMID: 38222607
- PMCID: PMC10785638
- DOI: 10.1021/acsomega.3c06544
Combined Experimental and Theoretical Insights: Spectroscopic and Molecular Investigation of Polyphenols from Fagonia indica via DFT, UV-vis, and FT-IR Approaches
Abstract
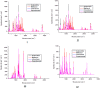
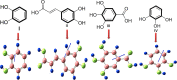
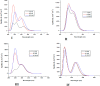
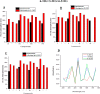
This review deals with computational study of polyphenolic compounds of medicinal importance and interest for drug development. Herein, four polyphenolic compounds comprising catechol (1), caffeic acid (II), gallic acid (III), and pyrogallol (IV) have been isolated from a medicinal specie, Fagonia indica, by applying silica gel column chromatography. These compounds were identified by using gas chromatography-mass spectrometry (GC-MS) analysis and confirmed by geometric computational analysis. According to computational results, caffeic acid has shown the highest biological activation due to higher chemical softness, electronegativity (χ (eV) = -648.644), and electrostatic potential value (-8.424 × 10-2 to +8.424 × 10-2), while smaller values of chemical potential (-0.269), ELUMO (-0.080), and energy gap (ΔE = 0.149). The Mulliken atomic charges were calculated by using DFT/B3LYP with basis set 6-311G for the determination of active sites. The oxygen atom of catechol showed highest nucleophilic characteristic with a more negative charge (08 = -0.695), and pyrogallol indicated a strong electrophilic center at C14 = 0.415 with a higher positive charge. Moreover, UV-visible absorption spectra and a detailed study of vibrational frequencies for all phenolic compounds by employing the DFT approach with 3-21G, 6-31G, and 6-311G basis sets at the ground-state level showed the great agreement with experimental results. ANOVA has been applied to validate the theoretical data. Results suggest that compounds I-IV are suitable in diverse fields.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
FT-IR, FT-Raman, NMR and UV-Vis spectra and DFT calculations of 5-bromo-2-ethoxyphenylboronic acid (monomer and dimer structures).Spectrochim Acta A Mol Biomol Spectrosc. 2015 Feb 25;137:1315-33. doi: 10.1016/j.saa.2014.08.049. Epub 2014 Sep 2. Spectrochim Acta A Mol Biomol Spectrosc. 2015. PMID: 25305625
-
Conformational analysis, spectroscopic study (FT-IR, FT-Raman, UV, 1H and 13C NMR), molecular orbital energy and NLO properties of 5-iodosalicylic acid.Spectrochim Acta A Mol Biomol Spectrosc. 2015 Feb 5;136 Pt B:295-305. doi: 10.1016/j.saa.2014.08.137. Epub 2014 Oct 29. Spectrochim Acta A Mol Biomol Spectrosc. 2015. PMID: 25448933
-
FTIR, FT-RAMAN, NMR, spectra, normal co-ordinate analysis, NBO, NLO and DFT calculation of N,N-diethyl-4-methylpiperazine-1-carboxamide molecule.Spectrochim Acta A Mol Biomol Spectrosc. 2013 Nov;115:275-86. doi: 10.1016/j.saa.2013.06.011. Epub 2013 Jun 20. Spectrochim Acta A Mol Biomol Spectrosc. 2013. PMID: 23845985
-
Antagonistic properties of a natural product-Bicuculline with the gamma-aminobutyric acid receptor: studied through electrostatic potential mapping, electronic and vibrational spectra using ab initio and density functional theory.Spectrochim Acta A Mol Biomol Spectrosc. 2011 Dec 15;84(1):144-55. doi: 10.1016/j.saa.2011.09.022. Epub 2011 Sep 16. Spectrochim Acta A Mol Biomol Spectrosc. 2011. PMID: 21968210
-
Experimental (FT-IR and FT-Raman), electronic structure and DFT studies on 1-methoxynaphthalene.Spectrochim Acta A Mol Biomol Spectrosc. 2011 Aug;79(3):646-53. doi: 10.1016/j.saa.2011.03.051. Epub 2011 Apr 8. Spectrochim Acta A Mol Biomol Spectrosc. 2011. PMID: 21530378
Cited by
-
Weighted gene coexpression network analysis and machine learning for the determination of tfh cell and B cell infiltrating biomarkers in thymoma-associated myasthenia gravis.Heliyon. 2024 Jul 10;10(14):e34364. doi: 10.1016/j.heliyon.2024.e34364. eCollection 2024 Jul 30. Heliyon. 2024. PMID: 39108902 Free PMC article.
References
-
- Iftikhar F.; Khan M. B. N.; Tehreem S.; Kanwal N.; Musharraf S. G. BCL11A-targeted γ-globin gene induction by triterpenoid glycosides of Fagonia indica: A preclinical scientific validation of indigenous herb for the treatment of β-hemoglobinopathies. Bioorg. Chem. 2023, 140, 106768.10.1016/j.bioorg.2023.106768. - DOI - PubMed
-
- Eman A. A.; Gehan H. A.; Yassin M.; Mohamed S. Chemical composition and antibacterial activity studies on callus of Fagonia arabica L. Acad. Arena 2010, 2 (12), 91–106.
-
- Fang Z.; Bhandari B. Encapsulation of polyphenols-a review. Trends Food Sci. Technol. 2010, 21 (10), 510–523. 10.1016/j.tifs.2010.08.003. - DOI
-
- Qi X.; Liu H.; Ren Y.; Zhu Y.; Wang Q.; Zhang Y.; Wu Y.; Yuan L.; Yan H.; Liu M. Effects of combined binding of chlorogenic acid/caffeic acid and gallic acid to trypsin on their synergistic antioxidant activity, enzyme activity and stability. Food Chem.: X 2023, 18, 100664.10.1016/j.fochx.2023.100664. - DOI - PMC - PubMed
-
- Bhootham V.; Gul M. Z.; Chernapalli V.; Rupula K. Polyphenol Profile in different Maize varieties and their Antioxidant Potentials: Implications in Disease Resistance. Biol. Forum—Int. J. 2023, 15 (5a), 441–449.
LinkOut - more resources
Full Text Sources
Miscellaneous
