Proteomics and Metabolomics Analysis Reveal the Regulation Mechanism of Linoleate Isomerase Activity and Function in Propionibacterium acnes
- PMID: 38222669
- PMCID: PMC10785318
- DOI: 10.1021/acsomega.3c08243
Proteomics and Metabolomics Analysis Reveal the Regulation Mechanism of Linoleate Isomerase Activity and Function in Propionibacterium acnes
Abstract
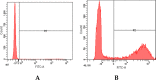
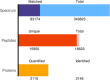
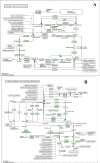
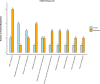
Conjugated linoleic acid (CLA) holds significant application prospects due to its anticancer, anti-atherosclerosis, lipid-lowering, weight-loss, and growth-promoting functions. The key to its efficient production lies in optimizing the biocatalytic performance of linoleic acid isomerase (LAI). Here, we constructed a Propionibacterium acnes mutant library and screened positive mutants with high linoleate isomerase activity. The proteomics and metabolomics were used to explore the mechanism in the regulation of linoleic acid isomerase activity. High-throughput proteomics revealed 104 differentially expressed proteins unique to positive mutant strains of linoleic acid isomerase of which 57 were upregulated and 47 were downregulated. These differentially expressed proteins were primarily involved in galactose metabolism, the phosphotransferase system, starch metabolism, and sucrose metabolism. Differential metabolic pathways were mainly enriched in amino acid biosynthesis, including glutamate metabolism, the Aminoacyl-tRNA biosynthesis pathway, and the ABC transporter pathway. The upregulated metabolites include dl-valine and Acetyl coA, while the downregulated metabolites include Glutamic acid and Phosphoenolpyruvate. Overall, the activity of linoleic acid isomerase in the mutant strain was increased by the regulation of key proteins involved in galactose metabolism, sucrose metabolism, and the phosphotransferase system. This study provides a theoretical basis for the development of high-yield CLA food.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Shenderov B. A.; Sinitsa A. V.; Zakharchenko M. M.; Lang C.. Metabiotics: Present State. In Challenges and Perspectives, 1st ed.; Springer Nature: Cham, Switzerland, 2020; pp 5–9.
-
- Dahiya D. K.; Puniya A. K. Optimisation of fermentation variables for conjugated linoleic acid bioconversion by Lactobacillus fermentum DDHI 27 in modified skim milk. Int. J. Dairy Technol. 2018, 71, 46–55. 10.1111/1471-0307.12375. - DOI
-
- Tapia A. M.; Bautista J. A. N.; Mendoza B. C.; Pham L. J.; Sarmago I. G.; Oliveros M. C. R. Production of conjugated linoleic acid by lactic acid bacteria: Screening and optimization. Philipp. J. Sci. 2019, 148, 457–464.
-
- Baliyan N.; Maurya A.; Kumar A.; Agnihotri V. K.; Kumar R. Probiotics from the bovine raw milk of Lahaul valley showed cis-9, trans-11 conjugated linoleic acid isomer and antioxidant activity with food formulation ability. LWT 2023, 176, 114553 10.1016/j.lwt.2023.114553. - DOI
-
- Ayivi R. D.; Gyawali R.; Krastanov A.; Aljaloud S. O.; Worku M.; Tahergorabi R.; Silva R. C. D.; Ibrahim S. A. Lactic acid bacteria: Food safety and human health applications. Dairy 2020, 1 (3), 202–232. 10.3390/dairy1030015. - DOI
LinkOut - more resources
Full Text Sources
