Immunological outcomes of autologous hematopoietic stem cell transplantation for multiple sclerosis: a systematic review
- PMID: 38222726
- PMCID: PMC10783339
- DOI: 10.1097/MS9.0000000000001490
Immunological outcomes of autologous hematopoietic stem cell transplantation for multiple sclerosis: a systematic review
Abstract
Background: Autologous hematopoietic stem cell transplantation (AHSCT) is an extensive procedure that allows for the depletion of the immune system and its restoration from hemopoietic stem cells. The approach has been modified for the treatment of severe immune-mediated illnesses, including multiple sclerosis (MS), after being initially devised for the treatment of hematological malignancies.
Objective: This systematic review aims to determine and consolidate the information on the short-term and long-term immunological effects of AHSCT on the cellular level in MS patients.
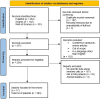
Methods: The PubMed, Scopus, and Web of Science servers were used to conduct a systematic search in compliance with the PRISMA guidelines. The results were tabulated and analyzed.
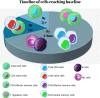
Results: A total of 17 studies (10 clinical trials, 6 cohort studies, and 1 case-control study) were included in the final analysis, and 383 MS patients were analyzed. A significant decline in the cell count of CD4 T cells was reported when compared to the CD8 T cells, B cells, and NK cells. B cell count returned to baseline in 71.4% of the studies at the end of 6 months. The NK cell count was found to be above the baseline in 62.5% of studies.
Conclusion: AHSCT has been proven to be one of the most effective treatment modalities for MS in recent studies. However, debilitating complications due to immunological outcomes of the procedure have led to increased morbidity. Further research into this domain will help boost the success rate and efficacy of AHSCT.
Keywords: autoimmune disorders; autologous hemopoietic stem cell transplant; immune cells; multiple sclerosis; myeloablation.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
None.Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Figures
References
-
- Dobson R, Giovannoni G. Multiple sclerosis – a review. Eur J Neurol 2019;26:27–40. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
